3/1/2020
Chasing pH in Wood Substrates
Jake C. Hudgins & Dr. Brian E. Jackson
Substrate pH has been and remains one of the critical variables in container crop production. The impact that pH plays on virtually all growing systems has been understood since container production began! We’ve learned how to manage pH before planting and at every stage of crop production when using the traditional organic substrate materials of peat, bark and coconut coir.
Now that wood products have entered the main stage of container production around the globe, the need to better understand (so we can predict and control) pH is upon us. To our estimation, concerns that growers have with wood mixes (when either trialing or adopting) are most centered around irrigation management and pH. Some background of wood pH and what we’re learning is discussed in the following.
Acidity
The natural acidity of wood can be attributed to several factors, including tree species, age of the tree, location of wood within the tree, site location and soil type trees are grown on, and season of tree harvest. The pH comes from the acidic nature of the tree components, in particular the organic acids (acetic, formic, oxalic) and polyphenols present in the wood.
As an example of pH ranges in wood, research on pine tree substrates (hammer-milled wood substrate) over the past 15 years has reported substrate pHs of 100% wood to range from 4.4 to 6.0—a range mostly attributed to the season of tree harvest. pH of wood can also be directly and significantly affected (changed) by post-harvest processing and treatment of wood chips, including exposure to external heat or steam, distillation, pressurized soaking in a pre-treatment bathing solution, chemical application or biochemical exposure/inoculation.
As an example, the pH of a freshly processed pine wood substrate component at a grower here in North Carolina in the spring of 2019 was 5.4, but when the wood material was dried in a force-air dryer, the pH dropped to 4.2. We’ve observed changes in wood pH as a result of drying many times over the years and if wood substrate materials are to be dried as part of their processing or storage protocols, close monitoring of the pH should be accounted for in lime applications at the time of substrate formulation.
Manufacturing method
Specifically pertaining to wood materials processed for substrate use, the manufacturing method employed to reduce wood chips into the final product can directly affect the pH of the end product. For example, the pH of pine wood chips hammer-milled will most likely not have a change in pH after processing into pine tree substrate. Conversely, pH has been shown to change (drop) in wood chips processed through thermomechanical processes like that of twin disc refiners when producing wood fiber.
Changes in wood substrate pH during storage has also been observed and reported in literature. Pine tree substrate (unamended) stored for one year can see the pH drop from 5.6 to 4.2. Such pH drops in other wood products hasn’t been investigated or reported.
Despite the pH drop, the low pH buffering capacity of wood materials makes it easy to increase the pH with low amounts of lime additions. There’s limited data and observations to date on pH of wood substrates, but there’s even less known about the pH of mixes containing different wood products.
pH trial
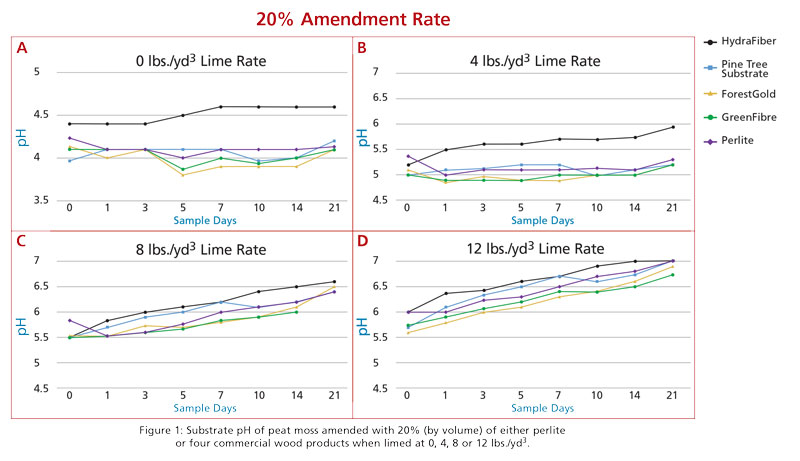
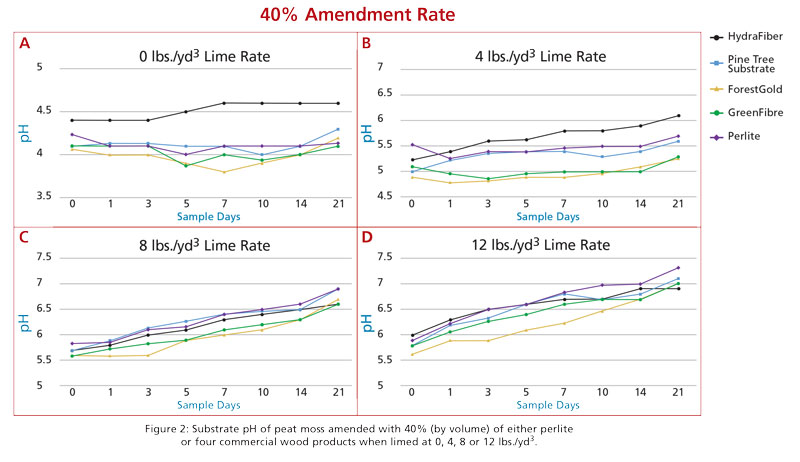
In response to this current lack of information, a project was initiated in the Horticultural Substrates Laboratory at NC State University in the summer of 2019 to evaluate the pre-plant substrate pH modification of Canadian sphagnum peat when blended with commercial wood substrates at varying rates and at increasing rates of dolomitic limestone additions. Horticultural grade peat moss was amended with eight wood products, including pine tree substrate, ForestGold, GreenFibre, HydraFiber, FloraFibre, HortiFibre, Topora, and pine wood chips. All wood products were blended with peat at ratios of 20%, 40%, 60%, 80% and 100%, plus perlite amendments of 20% and 40% (as controls) for a total of 42 substrate blends.
Each blend was wetted to 60% moisture content and subsamples were amended with 200 mesh dolomitic limestone at rates of 0, 4, 8 and 12 pounds/yard3. Samples were incubated in a controlled climate chamber and sampled for pH determination on zero, one, three, five, seven, 10, 14 and 21 days after lime addition.
For the purpose of this article, we’ll present and discuss data from the most common wood products currently in use in the U.S., which include pine tree substrate, ForestGold, HydraFiber, GreenFibre plus perlite at inclusion rates of 20% and 40%.
The pH of the peat moss used in this trial was 4.0 and the pH of the 100% wood substrates was 5.5 (pine tree substrate), 4.4 (ForestGold), 5.0 (HydraFiber) and 5.2 (GreenFibre). When peat was blended with 20% of those wood materials plus perlite (Figure 1), the resulting pH increased as the lime rate increased (as expected). All products were within half a pH unit (0.5) pretty consistently with the exception of the HydraFiber blend, which was a bit higher at the lower lime rates (0 and 4 pounds).
Based on these data and observations, if a grower blended most of these products in with peat at 20% they wouldn’t need to change their lime additions if their target pH range could be within 0.5 units. At lower lime rates, HydraFiber blends did tend to be higher. When peat was blended with 40% wood components, there was more separation in pH values for some of the different components (Figure 2). HydraFiber remained higher at the lower lime rates, but at the upper rates (8 and 12 pounds) most products were again within 0.5 units from each other. ForestGold and GreenFibre did tend to remain a bit lower compared to perlite and pine tree substrates.
These data may allow substrate manufacturers or growers to have more confidence in making decisions regarding lime additions when trying to set their substrate pH to a specific range prior to planting. It’s wise to note that the products tested could be tested again and yield different results due to differing sources of peat, age of wood material, mesh size of limestone, etc.
Potential variability in pH among different commercial wood substrate products, as well as possible variation of a specific product based on season or other variable, shouldn’t be of major concern. Wood is an organic material, and as such, its chemistry can change over time. This is similar to peat that’s well known to have variable pH across different bogs, within the same bog and at different times of the harvest season. Same for barks, coconut coir/fiber, compost and other organic materials whose age, handling and storage all affect its pH when used as (or in) a substrate.
We learn to manage substrate pH, not necessarily try to “permanently” change it. Manufacturers of wood substrate materials will learn how to properly adjust and maintain pH in mixes with high contents of wood and growers will subsequently learn to modify their practices to account for and control pH as needed.
Predicting, adjusting, monitoring and managing substrate pH will depend greatly on the percent wood product in the substrate, as well as what it’s amended with (peat, bark, coir or other materials). An improved knowledge of the acidic properties of wood fiber materials will be of benefit to the continued adoption, use and management of crops grown in substrates containing these components.
A better understanding of how to adjust pre-plant pH to a desired level for planting is very important for substrate manufacturers and those growers who make their own substrates, but how pH may change during crop production (and corrective measures) is the true challenge and applicable point of interest for most growers. Is the pH behavior similar to traditional mixes that don’t contain wood components? What percent of wood additions change pH during production? Do different wood products change pH differently over time? What’s the effect of water source and quality on pH? Effect of fertilizer type?
Currently, efforts are underway to address these unknowns, as well as others relating to pH and chemical properties for common crops including poinsettia, cannabis, marigold and petunia. GT
Jake Hudgins is an undergraduate student and Brian E. Jackson is an Associate Professor and Director of the Horticultural Substrates Laboratory at North Carolina State University. Brian can be reached at Brian_Jackson@ncsu.edu.