6/1/2018
Protect Your Christmas Poinsettias from Whiteflies
Steven Arthurs, Erfan Vafaie, Pete Krauter & Kevin M. Heinz
At this time of year, many growers are already planning their Christmas crop. Whether you grow your own stock plants or purchase unrooted cuttings, poinsettia propagation will yield desired results when starting with high-quality cuttings, maintaining optimal environmental conditions for propagation and concurrently minimizing pest issues. For best results, cuttings with two to three leaves of uniform size, caliper and maturity should be selected and rooted in Oasis Rootcubes or high-quality, peat-based media and maintained under a mist of purified water for three to four weeks.
During the early stages, cuttings require a constant film of moisture on the foliage, although the frequency and duration of misting can be gradually reduced as plants start to root. The media shouldn’t be saturated, as this will leach nutrients and delay rooting.
Unfortunately, the warm and humid conditions required for rooting cuttings can provide an ideal environment for pests and diseases. In the case of poinsettias, propagation under mist presents environmental and physiological challenges to managing whiteflies.
First, the residual control from a contact insecticide is limited by the frequent mist application. The lack of a developed root system also limits adsorption (uptake) from systemic insecticides. Whiteflies, however, are unhindered by the presence of mist and continue to lay eggs and develop on the leaves of cuttings. If cuttings are obtained from infested source plants, or if the propagation area is subject to new whitefly infestations, the new crop will begin its production cycle already infested! Other issues to watch out for during poinsettia propagation are fungus gnats, Botrytis and bacterial soft rot diseases.
Experiments with translaminar insecticides
Translaminar movement is the ability of the active ingredient of some pesticides to penetrate the leaf surface and move into the leaf tissue. Translaminar insecticides therefore offer a strategy to control whitefly during mist propagation. We tested insecticides with translaminar properties to manage Bemisia whiteflies on unrooted Prestige Red Poinsettia cuttings in a research greenhouse.
Whiteflies were artificially established on the stock plants first, so that cuttings in our tests came pre-infested with immature stages (eggs and young nymphs) of these pests. Whiteflies were also released under mist at the rate of one per cutting twice a week to simulate a worst-case scenario for the grower. We have observed whiteflies entering greenhouses from adjacent cotton fields following harvest of these fields.
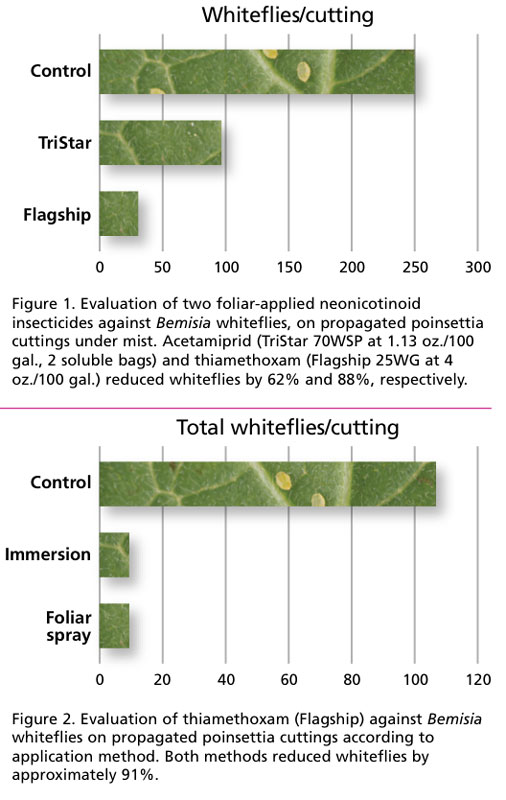
Experiment 1: Comparison of two insecticides
In the first experiment, we compared foliar applications of two neonicotinoid insecticides at label rates; i.e., thiamethoxam (Flagship 25WG) and acetamiprid (TriStar 70 WSP). Water was used for an untreated control.
Cuttings were rooted in 4-in. diameter pots containing Sunshine Grow Mix #1 potting media. Pots were placed on three mist benches and covered with a semi-transparent polyester fabric (Reemay) fitted over a 4-ft.-tall PVC frame to maintain humidity. On each bench, pots were randomly divided among the three treatments.
A decreasing misting cycle intensity was provided to harden the cuttings; i.e., 12 seconds every 12 minutes initially operated for 24 hours for three days, followed by operation only during daylight hours (6:00 a.m. until 8:00 p.m.) for a further 14 days, and finally, operation during only afternoon hours (12:00 p.m. until 4:00 p.m.) for a further eight days. Misting emitters (Phyto-Mist Brass Nozzles) were at
3-ft. spacing.
Insecticides were applied until run-off with spray bottles on day 11 of the misting cycle noted above (after misting had stopped). The study was terminated on Day 25 and whiteflies counted from all leaves in the laboratory with a dissecting microscope. Both insecticides reduced the number of whiteflies (see Figure 1).
Experiment 2: Comparison of application methods
The second study compared two application methods; i.e., a foliar application versus whole-plant immersion. The same rate of Flagship 25WG as the previous experiment was used in each case and control plants were left untreated.
For immersion, plants were submerged in a 5-gal. bucket for 10 seconds and then allowed to sit in a 69F (21C) room for one hour for absorption of the insecticide before planting. Foliar treatments were sprayed to “run off” 48 hours after planting after the mist system had ceased for the day.
Cuttings were rooted as before, this time with the misting cycle operated continuously for two days, followed by daytime operation for seven days and afternoon operation until the experiment was terminated on Day 24. Counts of whiteflies showed little difference between the methods (see Figure 2).
Conclusions
Managing pests early in production helps maintain a clean crop. Translaminar insecticides provide a tool to manage whiteflies during misting cycle of propagation when other insecticides may not work well. We didn’t observe any benefits of immersing cuttings rather than spraying. Currently, immersion or “dipping” isn’t an approved label use for thiamethoxam, so cuttings need to be sprayed.
In Canada, researchers and growers are experimenting with dipping poinsettia cuttings in soaps, oils and fungal-based biopesticides to kill whiteflies on infested propagated cuttings (see the April 2017 issue of GrowerTalks). This approach has an advantage, since some Bemisia whiteflies (the Q-biotype) are resistant to most chemical insecticides.
Currently, there’s no way to visually identify Q-biotype without a DNA test. Dipping cuttings in a bath (as opposed to spraying) also provides superior coverage for contact-based insecticides like soaps and oils, and may also result in less pesticide waste than spraying. However, you should check for phytotoxicity before treating large numbers of cuttings. It’s also a good practice to periodically change the dip bath to prevent the possible build-up of plant disease pathogens to infectious levels. GT
Steven Arthurs (Assistant Research Scientist), Erfan Vafaie (Doctoral Student and Extension Program Specialist), Pete Krauter (Senior Research Associate) and Kevin M. Heinz (Professor) work in the Department of Entomology at Texas A&M University in College Station, Texas. Dr. Arthurs is supported by a Texas A&M AgriLife Research Insect Vector Diseases Program and Dr. Heinz is regularly funded by the American Floral Endowment.