Whitefly on poinsettia
I’m going to start today’s newsletter with a little shameless self-promotion. The May issue of GrowerTalks is all about poinsettias. With tremendous help from my post-doctoral research fellow, Gunn Gill, my contribution to the issue is an article on controlling whiteflies. You can consider yourself a very selected lucky few if you grow poinsettias, but never suffer from whiteflies.
Before I talk about the article, I want to first talk about the terms “systemic” and “translaminar.” Some folks consider them the same thing, but “systemic” and “translaminar” are actually different levels of how easily the insecticide active ingredients move in plant tissues.
Let’s think about insecticide mobility in plant as a spectrum, like a rainbow’s color from red to violet. On one end of the spectrum is a non-systemic insecticide, which doesn’t move into plant tissues. On the other end of the spectrum is a systemic insecticide, which moves easily into plant tissues and can be transported through the plants via the xylem or phloem. A translaminar insecticide moves into leaf tissues, but the active ingredient typically stays in the tissues it enters.
Even within translaminar and systemic insecticides, there are differences in how “systemic” they are. (Let’s call this difference “systemicity.”) For example, I often think of dinotefuran (Safari) as being more “systemic” than imidacloprid (Marathon) because dinotefuran is more water-soluble and can move into and up the treated plants more easily and quickly. Sometimes that difference in systemicity can change how the insecticides are used. For example, pymetrozine (Endeavor) has greater systemicity than pyrifluquinazon (Rycar), so Endeavor can be used in drench applications, but Rycar cannot.
The article in the May issue of GrowerTalks, entitled “Warding Off Whiteflies,” discusses a few of my studies on how to control whiteflies on poinsettias effectively. The main goal of the article is to answer the question, “What can I use to replace neonicotinoids in controlling whiteflies?”
We started with a couple of studies demonstrating the effectiveness of dipping poinsettia cuttings in a mixture of Beauveria bassiana (BotaniGard) and insecticidal soap (M-Pede) before sticking. Then we discussed a study by Rose Buitenhuis of Vineland Research & Innovation Centre on how cutting dips can make biological control more effective. I cannot stop myself from recommending cutting dips to everyone because it’s very effective.
We then talked about a study conducted in my lab. In this study, we compared the efficacy of 12 different systemic and translaminar insecticides when they’re applied at label rate as a medium drench or foliar spray. The goal of this study was to identify products that are as effective as (if not more effective than) dinotefuran and imidacloprid in controlling whiteflies on poinsettia. There were some winners and some losers. Check out our article to find out.

How whiteflies deal with plant toxins
Here's more research with an interesting hypothesis on how whiteflies can deal with plant toxins. Richard Criley alerted me to this story. (Thanks, Richard!)
Plants and bugs are constantly at war—the plants produce chemicals that can repel or kill the bugs, and the bugs find ways to get around these defenses. I have to say, bugs really have it figured out! That advantage over plants helps some species tremendously.
Exhibit Number 1: The sweetpotato whitefly (also known as the silverleaf whitefly). Sweetpotato whitefly can feed on hundreds of different plant species, including nightshades. Nasty chemicals produced by nightshades can make mortals, like you and me, sick as a dog! For example, tomato plants produce phenolic glycosides, which is a major defense chemical against insects. How do whiteflies feed on tomato plants without getting sick?
Ted Turlings of the University of Neuchâtel in Switzerland and his colleagues have discovered a potential mechanism. In a paper published in the April issue of Cell, Turlings and his team found a gene known as BtPMaT1 in the genome of sweetpotato whitefly. What’s the significance of this gene? Well, BtPMaT1 is previously known only from plants. The gene codes for the production of phenolic glycoside malonyltransferase, which is an enzyme that helps plant neutralizes and stores the defensive chemical. The presence of BtPMaT1 means that the whiteflies can also produce the same enzyme and neutralize phenolic glycosides when they feed on tomato plants.
To demonstrate the importance of BtPMaT1 to the whitefly’s ability to feed on tomato, the researchers developed tomato plants that can produce a small RNA molecule that disrupts the function of BtPMaT1. Whiteflies that fed on these modified tomato plants weren't able to produce the detoxification enzyme and died.
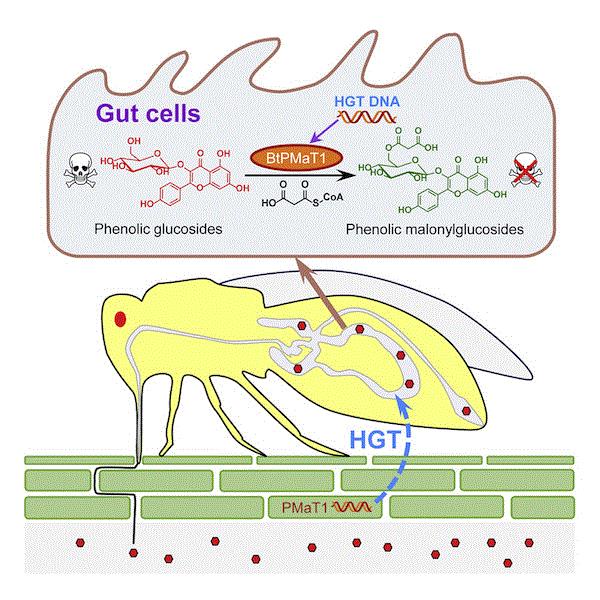
A schematic of how whitefly uses BtPMaT1 to detoxify plant toxins. (Source: Xia et al. 2021, Cell 184 [7]: 1693-1705.)
This is the first documented case of BtPMaT1 in insects. So how did a gene that was supposedly only present in plants ended up in an insect’s genome? The research proposed that horizontal gene transfer between a plant and an insect had made this possible. Horizontal gene transfer is a process by which genes are moved from one organism to another organism, often of a different species. (Gene transfer from parents to children is called vertical gene transfer.)
The acquisition of BtPMaT1 might have happened 35 to 80 million years ago when a plant virus picked up the gene and was then ingested by the ancestor of whitefly, which subsequently incorporated the gene in its genome.
Fascinating stuff! Read a brief of the study by clicking HERE.

Disinfectants vs. viruses
Staying on the theme of research, I want to introduce y’all to a recent study on virus management.
If you grow vegetables or are familiar with vegetable pest management, you probably could make a long list of viruses that affect tomatoes, cucurbits, brassicas, etc., right off your memory. Recent introductions, such as the tomato brown rugose fruit virus (ToBRFV) and cucurbit leaf crumple virus (CLCV), are attracting a lot of attention.
Managing these new viruses is understandably challenging. Even some of the “older” viruses, such as tomato spotted wilt virus (TSMV) and cucumber green mottle mosaic virus (CGMMV), have few management tools.
Insect-transmitted viruses, such as CLCV and TSWV, can be managed by reducing their insect vectors (thrips for TSWV and whiteflies for CLCV). Management of seed-borne viruses, such as ToBRFV and CGMMV, are more challenging because the viruses can be spread to uninfected seeds or seedlings simply by handling infected seeds. Once introduced into the field, the seed-borne viruses can be passed around or maintained through contacts with surfaces, such as farming equipment and debris.
Sanitation and disinfection are extremely important in reducing the transmission and persistence of seed-borne viruses. But what kind of disinfectants should you use? There are now some answers to this question thanks to recent studies by Kai-Shu Ling of USDA-ARS and his colleagues.
In a study published in Virology Journal, Dr. Ling and his colleagues evaluated the effectiveness of 16 disinfectants in reducing the transmission of ToBRFV and CGMMV. Some of the tested disinfectants are familiar household names, such as Purell, Kleengrow, Lysol and Clorox. The researchers mixed liquid containing the viruses with the disinfectant solution, then rubbed the mixture on seedlings (with the ever-versatile Q-Tip). The researchers then recorded how many of the seedlings were infected.
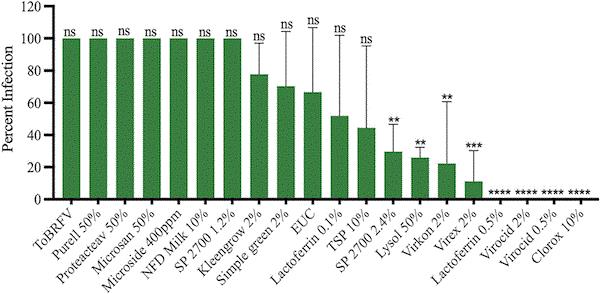
Percentage of tomato plants infected with tomato brown rugose fruint virus (ToBRFV) after exposure to virus inoculum treated with different disinfectants. Disinfectants topped with asterisks are the ones with significant reduction in percentage of infection compared to the inoculated control.
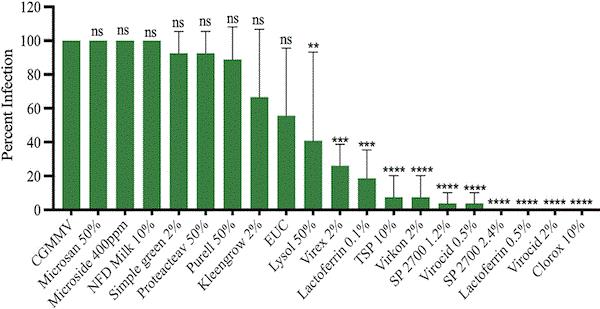
Percentage of watermelon plants infected with cucurbit green mottle mosaic virus (CGMMV) after exposure to virus inoculum treated with different disinfectants. Disinfectants topped with asterisks are the ones with significant reduction in percentage of infection compared to the inoculated control.
The disinfectants with 90% to 100% efficacy against both viruses are Lactoferrin (lactoferrin powder), Virocid (a mixture of alkyl dimethyl benzyl ammonium chloride, didecyl dimethyl ammonium chloride and glutaraldehyde), Clorox (sodium hypochloride) and Virkon (potassium peroxymonosulfate and sodium chloride). It’s important to use the right application rates of these effective disinfectants.
The most effective concentrations for managing ToBRFV are 0.5% Virocid, 10% Clorox, 3% Virkon and 0.5% Lactoferrin. For CGMMV, the most effective concentrations are 5% Clorox, 0.5% Lactoferrin and 2% Virocid. (Virkon wasn’t included in the concentration vs. CGMMV study.)
Other disinfectants I didn't mention here can also reduce the incidence of the two viruses, albeit at a lower efficacy than those mentioned above. Check out the article for more information.






See y'all later!
JC Chong
Professor of Entomology at Clemson University
This e-mail received by 26,607 subscribers like you!
If you're interested in advertising on PestTalks contact Kim Brown ASAP!