5/1/2021
Warding Off Whiteflies
JC Chong & Gunbharpur Gill

Had poinsettias been introduced during Benjamin Franklin’s time instead of 35 years after his death, Franklin would have complained, “When growing poinsettias, nothing can be said to be certain, except whitefly.” It’s often not a matter of if, but when whiteflies will have a feast on your poinsettia crop.
Pictured: Dipping poinsettia cuttings in 0.5% insecticidal soap and Beauveria bassiana (BotaniGard 22WP at 1 lb. per 100 gal.) or 0.1% SuffOil-X can significantly reduce whitefly populations.
JC was too young to be involved, but his mentor, Dr. Ron Oetting of the University of Georgia, identified the introduction of neonicotinoids in the mid-1990s through the early 2000s as a watershed moment in the history of whitefly management. Widespread use of neonicotinoids had relegated whiteflies to being a major, but manageable, pest of poinsettia and other ornamental crops.
Twenty years later, we’re at another watershed moment. This time, the value of neonicotinoids is being questioned in our industry. A lot of efforts have been invested in identifying and introducing alternatives to neonicotinoids.
Let’s take stock of the lessons we can apply to managing whiteflies in the upcoming poinsettia season. The new neonicotinoid-light or neonicotinoid-free whitefly management programs will have to truly integrate all cultural, chemical and biological control tools available.
Let’s start with the basics
The first basic fact about sweetpotato whitefly, which is the species responsible for most infestations on poinsettia crops in North America, is that whiteflies have to come from somewhere. One source could be a population hiding on weeds and stock plants inside the greenhouse, made possible by sweetpotato whitefly’s ability to feed on 500 or so plant species. Sanitation, including the removal of weeds, last season’s unsold crop (face it—you can’t sell them anymore) and pet plants in and around the greenhouse, is important in minimizing infestation from this internal source. Whitefly populations on stock plants should be kept at the lowest level possible with biological control or insecticide applications (more about this later).
If you’ve done so well with sanitation that you can literally eat off the floor (although we don't recommend it), but you still suffer from whiteflies, then you need to consider external sources of whiteflies. Sweetpotato whitefly couldn’t overwinter outside of a greenhouse north of South Carolina. It’ll take time for the whiteflies to migrate from their permanent habitat in the Southern U.S. and build up a population in the fields through the spring and summer. Wind carries some adults from these outdoor populations into greenhouses with open vents or sides. These invading whiteflies must be detected with yellow sticky cards and can be stopped by screening doors and vents.
Start clean by dipping cuttings
If whitefly infestation occurs despite good sanitation and screening, they may have been introduced on infested cuttings. Incoming cuttings should be inspected carefully for whiteflies and other pests before sticking. Whitefly eggs and nymphs are small, so pre-planting inspection may not catch all of them. We recommend dipping cuttings (rooted or unrooted, purchased or propagated in-house) in a mixture of 0.5% insecticidal soap (M-Pede) and Beauveria bassiana (Botanigard) or 0.1% SuffOil-X. The concentrations of soap and oil solutions have to be much lower than the regular spray rate to avoid phytotoxicity while not losing efficacy.
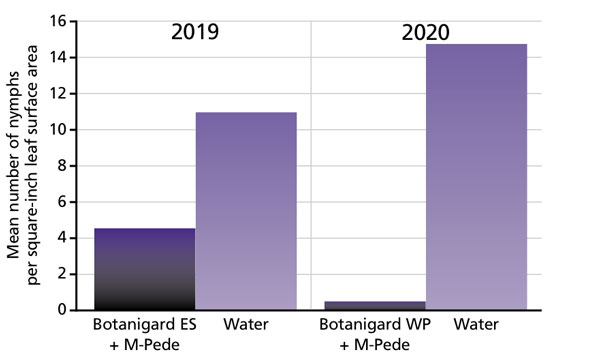 Table: Cutting dip with a mixture 0.5% M-Pede and Botanigard
Table: Cutting dip with a mixture 0.5% M-Pede and Botanigard
(ES formulation at 32 fl. oz. per 100 gal. and 22 WP formulation at 64 oz. per 100 gal.) was effective in reducing whitefly numbers on poinsettias in studies conducted by JC Chong. Cutting dip of Botanigard ES alone caused severe phytotoxicity, and therefore, is not recommended.
Studies by Dr. Rose Buitenhuis and colleagues at Vineland Research and Innovation Centre in Canada demonstrated that dipping cuttings killed 69% to 70% of whiteflies. Our own studies showed that poinsettia plants grown from cuttings dipped in a mixture of Botanigard and M-Pede (insecticidal soap) harbored 60% to 97% fewer whiteflies than cuttings dipped in water eight weeks after sticking. We recommend using Botanigard 22 WP instead of Botanigard ES because the latter has a greater chance of causing phytotoxicity. (Cutting dip is easy and you can find a video from Vineland on how to do it on their YouTube channel.) A lower starting population of whiteflies on dipped cuttings will give you weeks of breathing room before additional treatment is required.
Dipping & biologicals
Cutting dip can make biological control more effective. Another study by Dr. Buitenhuis found dipped cuttings that also received parasitoids had almost eight times fewer whiteflies than no treatment and four times fewer whiteflies than a dip-only treatment at seven weeks after sticking. Once again, cutting dip reduces whitefly abundance on cuttings to a low enough level that the parasitoids are able to do their best.
Parasitoids (Eretmocerus for sweetpotato whitefly and Encarsia for greenhouse whitefly) aren’t the only biological control agents available for releases. Predatory mites (Amblyseius swirskii and Amblydromalus limonicus), ladybeetle (Delphastus catalinae), predatory bug (Dicyphus hesperus), lacewings, and insect pathogens (Beauveria bassiana and Isaria fumosorosea) are also used.
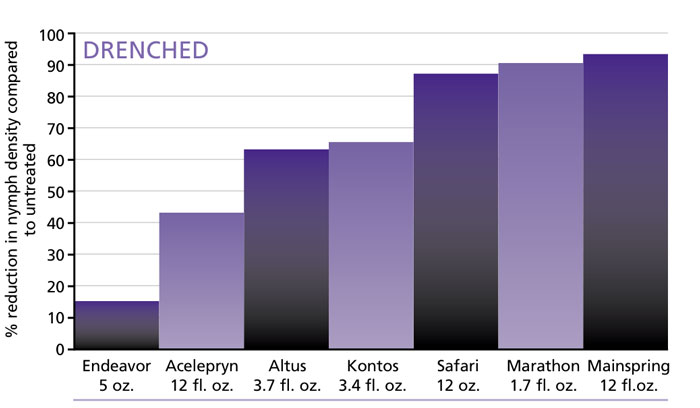 Table: Efficacy of drenched insecticides against whitefly nymphs at eight weeks after treatment. Application rates are on a per 100-gallon basis, except for Kontos and Marathon, which are on a per 1,000-pot basis.
Table: Efficacy of drenched insecticides against whitefly nymphs at eight weeks after treatment. Application rates are on a per 100-gallon basis, except for Kontos and Marathon, which are on a per 1,000-pot basis.
A biological control program against whiteflies on poinsettia is one of the most touted success stories. Many poinsettia stock plants and crops in the United States and Canada are protected solely by biological control. The keys to successful biological control are to start the program soon after sticking, and to plan ahead for regular shipments and releases of biological control agents.
Neonic replacements
While many operations have found success with biological control, others continue to produce beautiful poinsettia crops by protecting them with insecticides. Traditionally, many operations start with a medium drench of neonicotinoid (dinotefuran, imidacloprid or thiamethoxam) followed by foliar sprays to stamp out hot spots. In a neonicotinoid-free or neonicotinoid-light production, what can be used as replacements for neonicotinoids? There are several options for drench and foliar spray, but surprisingly, there’s no side-by-side comparison of all these options.
We set out to gather that information in 2019 by conducting an experiment to evaluate the efficacy of insecticides applied as drench and/or spray at label rates againstB-biotype sweetpotato whitefly on a poinsettia crop. The neonicotinoid alternatives evaluated include afidopyropen (Ventigra; IRAC 9D), chlorantraniliprole (Acelepryn; 28), cyantraniliprole (Mainspring; 28), cyclaniliprole (Sarisa; 28), flonicamid (Aria; 29), flupyradifurone (Altus; 4D), pymetrozine (Endeavor; 9A), pyrifluquinazon (Rycar; 9A), spinetoram + sulfoxaflor (XXpire; 5 + 4C), and spirotetramat (Kontos; 23). These insecticides were compared to dinotefuran (Safari 20SG; 4A) and imidacloprid (Marathon II; 4A), also applied as a spray and drench at label rates.
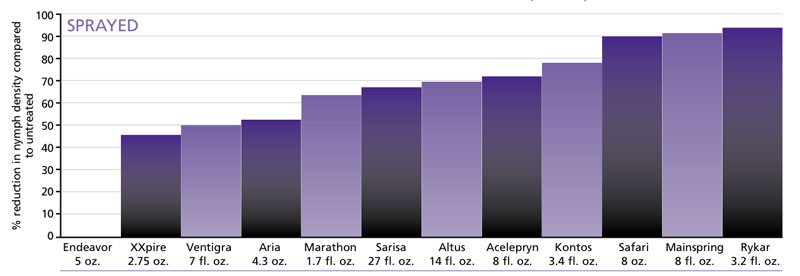 Table: Efficacy of sprayed (twice) insecticides against whitefly nymphs at eight weeks after the first treatment. Application rates are on a per 100-gallon basis.
Table: Efficacy of sprayed (twice) insecticides against whitefly nymphs at eight weeks after the first treatment. Application rates are on a per 100-gallon basis.
The selected insecticides are either systemic or translaminar, and have been registered for drench and spray applications, or spray application only. Drench application was applied once at 4 fl. oz. solution per 6-in. pot. Foliar sprays were done twice, 14 days apart, with Capsil (at 6 fl. oz. per 100 gal.) mixed in all spray solution. Each treatment was replicated on four plants. Each plant received either spray or drench treatment of one insecticide. We documented the numbers of whitefly nymphs and adults on three leaves per plant once a week for eight weeks.
So which neonicotinoid alternative performed as well as or better than dinotefuran or imidacloprid? Among products registered for drench, only Mainspring matched the efficacy of Marathon and Safari at eight weeks after treatment. Altus and Kontos reduced densities of nymphs by around 65% compared to the untreated control. Acelepryn, which is recently registered for greenhouse uses but not for whiteflies, achieved 43% reduction.
Among the spray applications, Mainspring, Rycar and Safari achieved greater than 90% reduction in nymph densities at eight weeks after the first treatment. Acelepryn, Altus, Aria, Kontos, Marathon, Sarisa and Ventigra achieved between 50% and 80% reduction.
Oddly, Endeavor applied by both methods was the poorest performing product in this experiment. While we observed reduction in whitefly densities immediately after spray application of Endeavor, we also saw a quick rebound of whitefly populations on Endeavor-treated plants. This observation suggested to us that when spraying Endeavor, as well as other insecticides, more than two appli-cations might be necessary to provide long-term suppression of the whitefly population.
When we develop a chemical management program for whiteflies, we need to think about ways to reduce the chance of insecticide resistance development. As demonstrated by the emergence of the Q-biotype, the sweetpotato whitefly has an amazing ability to develop resistance to frequently used insecticides. We need to rotate insecticides of different modes of action or IRAC numbers. If you use an insecticide for drench, you can no longer use insecticides of the same mode of action in subsequent sprays.
An insecticide treatment and rotation program requires planning and knowledge of available tools. Plan now and have a whitefly-free poinsettia season! GT
JC Chong is a Professor and Extension Specialist in Turf and Ornamental Crop Entomology, and editor of the PestTalks e-newsletter, and Gunbharpur Gill is a postdoctoral research fellow at Clemson University.