4/1/2023
Insect Growth Regulators
Raymond A. Cloyd & Nathan J. Herrick

There are different types of insecticides commonly used in greenhouse production systems to manage populations of insect pests. One type of insecticides are insect growth regulators.
Pictured: Insect growth regulators are active on the immature stages of insect pests such as whiteflies. Photos by Raymond Cloyd.
This article discusses the different types and modes of action of insect growth regulators commercially available for use in greenhouse production systems. However, before discussing insect growth regulators, it’s important to understand some basic insect physiology, which will help greenhouse producers know how insect growth regulators affect insect pests.
Insect physiology
There are two hormones responsible for regulating insect growth: ecdysone and juvenile hormone. The hormone ecdysone regulates when insects molt, whereas juvenile hormone regulates the body form of the insect after molting (ecdysis).
The shedding of the old skin (integument) and formation of a new skin is called molting. The molting process is controlled by hormones. Molting occurs when a signal is sent to the brain of an insect, resulting in the production of the prothoracicotrophic hormone (PPTH). The hormone is released into the blood (hemolymph) of an insect, which stimulates the prothoracic glands to release ecdysone into the blood. Ecdysone is then hydroxylated, which involves introducing a hydroxyl group (-OH) to a compound, resulting in the formation of a new compound called 20 hydroxyecdysone or 20E. The molting process is initiated when levels of 20E are increased.
Insect growth regulators
Insect growth regulators are insecticides that disrupt the molting process or modify growth and development of insects. Insect growth regulators do not directly cause insect mortality, but interfere with the normal mechanisms of development, resulting in insects being killed before they can become adults. Insects are typically killed in three to 14 days. However, the speed of insect death depends on the insect growth regulator, label rate used and life stage of the target insect pest. Insect growth regulators primarily manage insect pest populations by reducing the numbers over time, not immediately. Consequently, the number of individuals decreases in subsequent generations.
 Pictured: Some insect growth regulators can indirectly affect the reproductive ability of female whiteflies.
Pictured: Some insect growth regulators can indirectly affect the reproductive ability of female whiteflies.
Insect growth regulators may negatively affect the development of eggs, larvae, nymphs and/or pupae. Some insect growth regulators may cause insects to cease feeding before dying. In addition, insect growth regulators may negatively influence reproduction and egg viability (live larvae emerging or eclosing from eggs). The timing of insect growth regulator applications is important to maximize their effectiveness in managing insect pest populations.
Applications of insect growth regulators must be initiated when the most susceptible life stage is present. For example, many insect growth regulators are more active on the early life stages (1st and 2nd instars) than the later life stages (3rd and 4th instars). In general, insect growth regulators are more effective against insect pests that undergo complete metamorphosis (egg, larva, pupa and adult) than insect pests that undergo simple, incomplete or gradual metamorphosis (egg, nymph and adult).
Target insect pests and modes of action
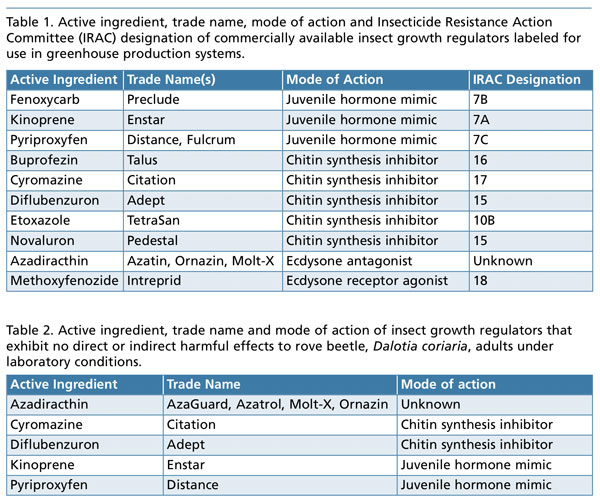
The target insect pests of insect growth regulators include: aphids, whiteflies, mealybugs, scales, fungus gnats, shore flies, leafminers, thrips and caterpillars. There are four different modes of action associated with insect growth regulators. (The insect growth regulators commercially available and their modes of action are listed in Table 1.) Here are descriptions of each insect growth regulator mode of action:
1. Juvenile hormone mimics/analogs: Inhibit development, thus causing insect pests to remain in immature (young) life stages, which prevents insect pests from completing their life cycle.
2. Chitin synthesis inhibitors: During the molting process, interfere with enzymes that stimulate the formation of chitin, which is an important component of an insect’s exoskeleton (cuticle). Consequently, insect pests fail to develop into adults because they die as immatures or they mature into sterile adult females.
 Pictured: Certain insect growth regulators are effective in managing populations of fungus gnat larvae.
Pictured: Certain insect growth regulators are effective in managing populations of fungus gnat larvae.
There are differences in the mode of action of chitin synthesis inhibitors depending on the active ingredient.
• Diflubenzuron (Adept): Inhibits the production of chitin, which is a protein-like compound responsible for the strength and resilience of the exocuticle (middle layer of the cuticle).
• Buprofezin (Talus): Prevents the levels of 20E from declining during initial molting (first life stages). If 20E levels are high during the molting process, then the old cuticle (skin) will not be digested and the new cuticle will fail to develop properly.
• Cyromazine (Citation): Causes the cuticle to be rigid (hard) or stiff, resulting in a decrease in elasticity, which reduces the ability of insect pests to move and feed.
3. Ecdysone antagonists: Disrupt or interfere with the molting process of insect pests by inhibiting metabolism of the molting hormone, ecdysone.
4. Ecdysone receptor agonists: Induce a premature molt by mimicking the activity of the molting hormone, ecdysone, which causes insect pests to cease feeding three to 14 hours after ingesting the compound.
Indirect effects on insect pests
Insect growth regulators primarily directly affect the immature (young) life stages of insect pests. However, some insect growth regulators may indirectly affect the adult life stage of certain insect pests by reducing reproduction or egg viability.
Here are examples of label information from insect (and mite) growth regulators associated with indirect effect on adults:
• Buprofezin (Talus): Suppresses egg-laying (oviposition) of adult females and reduces egg viability.
• Etoxazole (TetraSan): Possesses transovarial activity, which means that any treated adult female mites will not produce viable eggs.
• Pyriproxyfen (Distance): Reduces production of viable eggs associated with whitefly females due to transovarial activity.
Insect growth regulators and resistance
Similar to other insecticides, it’s important to rotate insect growth regulators with different modes of action to mitigate the potential for resistance developing in insect pest populations. Examples of insect pests that have developed resistance to insect growth regulators include green peach aphid, Myzus persicae, populations resistant to azadiracthin (e.g., Azatin, Ornazin, Molt-X) and sweetpotato whitefly, Bemisia tabaci, populations resistant to buprofezin (e.g., Talus), and pyriproxyfen (e.g., Distance). Therefore, insect growth regulators with the same mode of action should not be used in succession.
Direct effects on biological control agents
In our research at Kansas State University, we’ve found that certain insect growth regulators have no direct or indirect effects on rove beetle (Dalotia coriaria) adults under laboratory conditions. Table 2 lists the insect growth regulators that exhibit no direct or indirect harmful effects to rove beetle adults.
Summary
Insect growth regulators are a diverse group of insecticides classified into four distinct categories based on mode of action. Insect growth regulators can effectively manage immature (larva or nymph) populations of many different types of insect pests in greenhouse production systems. Therefore, insect growth regulators should be incorporated into insecticide rotation programs. However, timing of application is important because susceptible life stages or instars must be present for insect growth regulators to effectively manage insect pest populations. GT
Raymond A. Cloyd (rcloyd@ksu.edu) is a Professor and Extension Specialist in Horticultural Entomology/Plant Protection and Nathan J. Herrick (nherrick@ksu.edu) is a Research Associate in the Department of Entomology at Kansas State University in Manhattan, Kansas.