1/1/2022
Be Watchful of Your Water
Dr. Paul Fisher

The “best” fertilizer differs between growers. That’s because your irrigation water quality adds ions (nutrients such as calcium and magnesium, or other ions such as sodium, chloride and bicarbonates) into the nutrient solution coming out of the irrigation line. Ions from both irrigation water and fertilizer result in the final nutrient solution. This article emphasizes three concepts: 1) reduce your fertilizer nutrients if they’re already provided in irrigation water; 2) higher alkalinity water needs more ammonium in the fertilizer and/or acid injection; and 3) water test on a regular basis.
Figure 1. Tip burn in lettuce from low calcium.
Ions in irrigation water
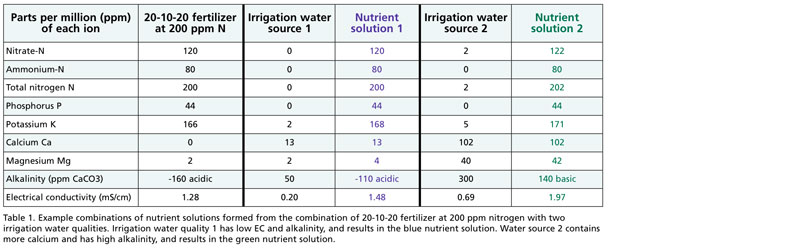
Table 1 provides the example of 20-10-20, which is a common water-soluble fertilizer. Note that 20-10-20 contains no calcium or negligible magnesium.
Irrigation water source 1 has low electrical conductivity (EC, a measure of total dissolved ions) and is a fairly pure water source. If 20-10-20 is combined with water source 1, the nutrient solution would contain low calcium (13 parts per million or ppm of Ca) and only 2 ppm of magnesium (Mg). That might be okay for short-term crops if the grower is using a peat-based substrate that contains dolomitic limestone as a source of Ca and Mg. However, if the grower is using a more inert substrate, such as coconut coir, and growing crops sensitive to calcium deficiency, 20-10-20 would be a poor choice.
Low calcium can lead to symptoms such as tip burn in lettuce (Figure 1), blossom end rot in tomato and pepper, or bract edge burn in poinsettia. If your water is like source 1, use a fertilizer such as 14-4-14 or 15-5-15 that contains adequate levels of calcium and magnesium.
In contrast, irrigation water source 2 contains moderate levels of 102 ppm Ca and 40 ppm Mg, which makes this water source a good match for 20-10-20.
Balance water alkalinity with nitrogen form and acid injection
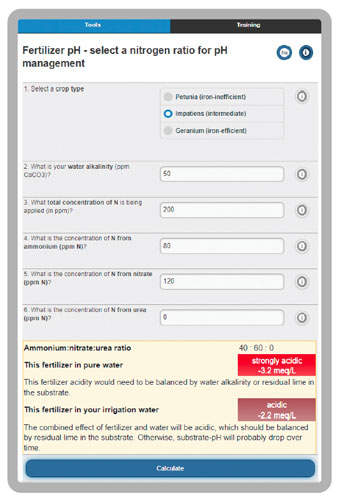
20-10-20 contains 40% of total nitrogen (N) as strongly acidic ammonium and 60% of N as mildly basic nitrate. Acid factors drop pH and basic factors raise pH. We can estimate the acidity of a fertilizer in different ways, but in general, any fertilizer with more than 20% of N as ammonium is likely to drop root zone pH. This pH drop occurs from the acidic processes of ammonium uptake by roots and nitrification of ammonium by soil microbes. We can estimate the acidity or basicity of a fertilizer in combination with water and plant species, which is 160 ppm of CaCO3 acidity for 20-10-20 at 200 ppm N (see the online tool at backpocketgrower.org/cce2.asp for more details, Figure 2).
Figure 2. The “Nitrogen form effect on pH” tool at backpocketgrower.org.
The water sources differ in alkalinity, which in water source 1 is low (50 ppm CaCO3), but is high (300 ppm CaCO3) in water source 2. Alkalinity can be thought of as dissolved lime. A moderate alkalinity of 100 ppm CaCO3 is equivalent to units of 122 ppm HCO3- (“bicarbonates”) or 2 milliequivalents/liter. Irrigating with high alkalinity is like liming your substrate (growing medium), leading to an increasing substrate-pH over time. In contrast, low alkalinity water can often lead to rapid pH changes in the substrate, often leading to low substrate-pH.
For water source 1, the acidity of 20-10-20 from ammonium would overpower the low alkalinity of the irrigation water and may lead to a drop in substrate-pH over time. The net effect of water alkalinity + fertilizer would be -160 ppm CaCO3, which is highly acidic. This could lead to issues such as iron/manganese toxicity at low substrate-pH (Figure 3). A high nitrate, low-ammonium fertilizer such as 13-2-13 or 15-5-15 is needed to balance this irrigation water source.
In contrast, the acidity of 20-10-20 would help balance the high water alkalinity of water source 2. However, the net effect of 140 ppm CaCO3 alkalinity is still fairly high and acid injection is likely to be needed. You can use the online Alkcalc tool developed by the E-Gro team (e-gro.org/alkcalc) to estimate the amount of mineral acid needed to control alkalinity for your water source.

Regular water testing
Send an irrigation sample for complete laboratory analysis of ions in your irrigation water at least annually and more often if your irrigation water changes seasonally. With even top-notch growers, this is one of those “important, but not urgent” tasks that’s often set aside. Use any of the major commercial or extension labs that handle greenhouse horticulture samples to ensure they include plant nutrients, such as calcium and magnesium, and other ions such as alkalinity, sodium and chloride.
Figure 3. Iron/manganese toxicity symptoms in older leaves is a common issue in iron-efficient crops, such as seed geranium, at low substrate-pH.
WaterQual is a free online tool to interpret your water test results, available at cleanwater3.org under “Tools.” Enter your water analysis into WaterQual and receive a customized report highlighting issues.
There are many other fine details on water quality and fertilizer choice. For more training on these topics, enroll in our online grower courses at hort.ifas.ufl.edu/training. GT
Dr. Paul Fisher is a Professor and Extension Specialist in Floriculture at the University of Florida. He can be reached at pfisher@ufl.edu.