10/1/2021
A Dynamic Duo
George Grant
First, get a water test
Testing your irrigation water can help determine an appropriate fertilizer program that sufficiently meets nutritional requirements while managing optimal substrate media pH. There are many labs available that offer water quality testing.
Regardless of the lab you choose, make sure to measure some specific horticultural parameters. These include pH, alkalinity, electrical conductivity (EC), primary (i.e., nitrogen, phosphorus, potassium) and secondary (i.e., calcium, magnesium, sulfur) plant macronutrients, as well as micronutrients such as iron. Sodium and chloride, which can negatively affect plant growth in large quantities, are also valuable to measure.
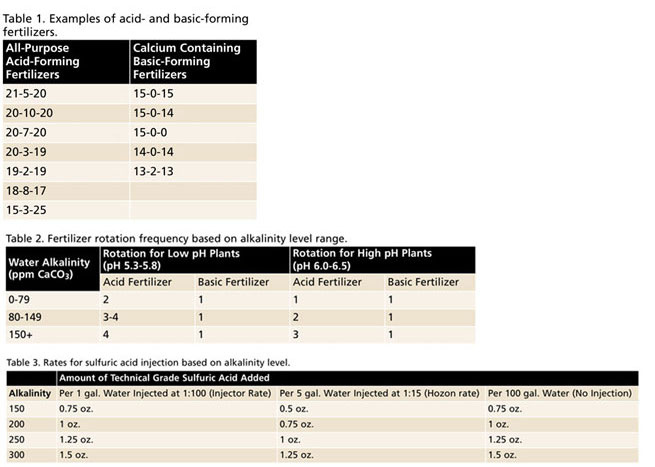
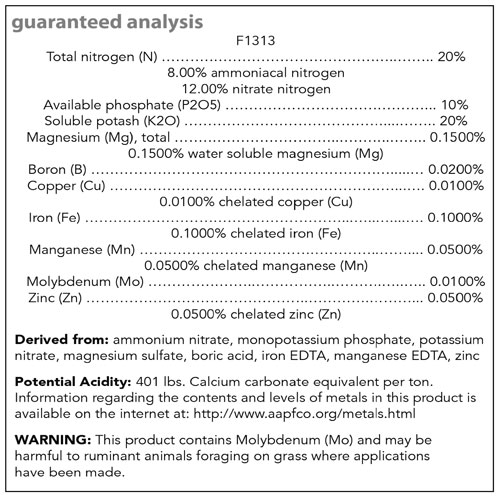
Figure 1. Example fertilizer label for Jack’s 20-10-20.
The quality of your “clear water” or water directly from your main irrigation source (e.g., wells, ponds, city water, etc.) without any additional inputs (e.g., fertilizer, acids, bases) should be determined year to year, as water quality can change, even seasonally. At a minimum, it’s a good idea to have your water tested annually.
The relationship between alkalinity and substrate pH
Alkalinity is a measure of the dissolved bicarbonates and carbonates (typically associated with calcium, magnesium and sodium) in water. If calcium and magnesium bicarbonate sound a lot like limestone to you, that’s because they are! Unless steps are taken to mitigate excessive alkalinity, the accumulation of these bicarbonates in the growing media will cause soil pH to rise. This will in turn affect the availability of essential nutrients for the plants, as most of them become less available as soil pH rises.
 Figure 2. Iron and manganese toxicity due to low soil pH in geranium.
Figure 2. Iron and manganese toxicity due to low soil pH in geranium.
Alkalinity can also be described as buffering capacity or resistance to pH change. Low alkalinity water offers little or no resistance to pH changes coming from acids (e.g., sulfuric acid) and fertilizers. High alkalinity water overwhelms or lessens the effects of acids and bases, and becomes the controlling factor of media pH.
How fertilizer affects substrate pH
Fertilizers can have a significant impact on raising or lowering substrate pH. The relative “acid-forming” or “basic-forming” ability of a given fertilizer is primarily determined by the percentage and/or ratio of ammonium and nitrate in the fertilizer.
For example, fertilizers with high amounts of ammonium (e.g., 21-7-7) are considered acid-forming fertilizers, whereas fertilizers containing predominately nitrate forms of nitrogen (e.g., 15-0-15) are considered basic-forming. The relative acid/basic-forming ability or “Potential Acidity or Basicity” of a fertilizer can typically be found on most fertilizer labels below the guaranteed analysis (Figure 1). Please reference Table 1 for commonly used examples of acid- and basic-forming fertilizers.
Bringing alkalinity back into the conversation, we can now combine our understanding of how both alkalinity and fertilizer choice work together to influence substrate pH. Each plant species has an optimal substrate pH in order for all the required macro and micronutrients to be available for plant uptake.
 Figure 3. Iron deficiency due to high soil pH in calibrachoa.
Figure 3. Iron deficiency due to high soil pH in calibrachoa.
Poinsettias, for example, have an optimal substrate pH of 6.0 to 6.5. If we go below or above this range, micronutrients can become overly available (i.e., nutrient toxicity, Figure 2) or unavailable (i.e., nutrient deficiency, Figure 3) for plant uptake, respectively. We can manage substrate pH by rotating between acid- and basic-forming fertilizers in an appropriate schedule based on the alkalinity of the irrigation water. As shown in Table 2, a poinsettia grower with an alkalinity level of 100 ppm CaCO3 would irrigate their crop twice with an acid-forming fertilizer and then use a basic-forming fertilizer on the third irrigation.
Adjusting substrate pH with acid
For water with alkalinity greater than 150 ppm CaCO3, injection of technical grade sulfuric acid is recommended to neutralize some amount of alkalinity and help keep media pH within the optimum range. When mixing up concentrated or “stock tank” fertilizer solutions, sulfuric acid may be added to most acid-forming fertilizers (e.g., 20-10-20 or 21-5-20), but isn’t compatible with any fertilizer containing calcium (e.g., 15-0-15, 15-0-0), which are typically basic-forming as well. Reference Table 3 for rates of continuous sulfuric acid injection based on alkalinity level.
Please feel free to reach out to Griffin’s technical team, GGSPro, for recommended rates when using other types of acids (e.g., phosphoric and citric), suggestions on where to have your water tested or if you have any questions on fertilizer rotation programs. GT
George Grant is a Technical Specialist for GGSPro.