6/30/2025
Through the Pores
Jeb S. Fields & Kristopher S. Criscione
Everyone you meet tends to have a different idea about proper plant nutrition and that’s actually fair. More than most production parameters, nutrition is influenced by many other aspects of your specific cultural practices. Environmental conditions play a major role in proper fertilizer application. Water management and temperature tend to be major drivers for fertilizer release from controlled-release fertilizers. Even the specific type of plant will dictate the type and quantity of fertilizer you apply. However, as with many other inputs, the pores themselves serve as managers, controlling how individual fertilizer salts or ions move through the profile of the container.
Pore Science II: Nutrition
As described in the first part of this series, we can think of the substrate as a “storage tank” for water, air and even roots. This storage tank can never be completely full of water (i.e., saturated); however, when all the substrate pores that can retain water are filled (i.e., container capacity), we consider this storage tank to be “full.” Horticulturists are continuously managing and refilling this tank with irrigation or fertilizer applications to provide resources to the plants. Proper management of this tank will ensure a healthy, high-quality crop. Of course, we want to refill these pores with water or nutrients at the proper rate, trying not to over-apply based on the specific capacity of the substrate. Otherwise, we lose any excess water and nutrients. This principle is true for all substrate-applied agrichemicals, such as fungicides and insecticides.
 Regardless of using water-soluble or controlled-release fertilizer, when there’s sufficient moisture, soluble mineral nutrients move across the pores via a concentration gradient (e.g., nutrients move from an area of high concentration to an area with fewer nutrients), so roots can access these nutrients. However, as moisture content decreases, be it from plant use or evaporation from the substrate on a hot and sunny day, both water and nutrients can become isolated and unavailable. In other words, proper water management is critical in ensuring that nutrients are available for root uptake and that they can still be mobile through the pores. Any nutrients stored in these inaccessible pores outside or below the root ball will be lost upon the next irrigation or storm event.
Regardless of using water-soluble or controlled-release fertilizer, when there’s sufficient moisture, soluble mineral nutrients move across the pores via a concentration gradient (e.g., nutrients move from an area of high concentration to an area with fewer nutrients), so roots can access these nutrients. However, as moisture content decreases, be it from plant use or evaporation from the substrate on a hot and sunny day, both water and nutrients can become isolated and unavailable. In other words, proper water management is critical in ensuring that nutrients are available for root uptake and that they can still be mobile through the pores. Any nutrients stored in these inaccessible pores outside or below the root ball will be lost upon the next irrigation or storm event.
Growing roots access their nutrients when our slow-release fertilizer releases encapsulated elements into the pore solution.
Looking deeper into the pores, both soils and substrates can have positive or negative electrical charges associated with them, much like a magnet. Nutrients are stored in the substrate by being adsorbed (e.g., sticking—like magnets) to the particle surface. When stuck nutrients are bumped off for other nutrients, we call this exchanging. Substrate components exchange nutrients to a much lesser degree than mineral soils. For instance, most of our substrates primarily contain a negatively charged surface. Since opposites attract, positively charged nutrients (called cations) often adhere to substrate particles. Cation exchange capacity (CEC) is the ability of a substrate, with negatively charged surfaces, to retain or exchange important positively charged ions that plants need such as potassium (K+), magnesium (Mg2+), ammonium (NH4+), iron (Fe2+) or calcium (Ca2+). Over time, CEC of the substrate can act as a small reservoir for cations to be held and exchanged. However, since we constantly apply fertilizers to the rootzone, this isn’t a primary storage reserve.
To a much lesser degree, substrate components can have anion exchange capacity (AEC), which is the ability of a positively charged substrate surface to retain or exchange negatively charged ions (anions) like some phosphorus (P) or boron (B). These nutrients exist in water as oxyanion (i.e., surrounded by oxygen forms), phosphate (PO4-) or borate (BO2-). Other anions like nitrate (NO3-) of chlorine (Cl-) have little affinity or strength to bind to substrate surfaces.
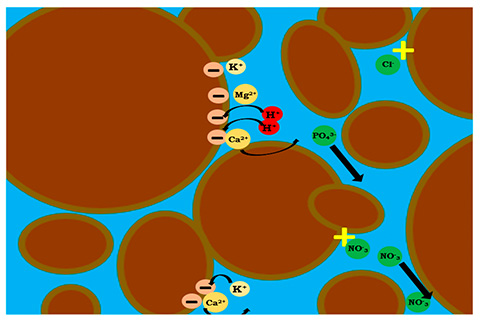 Revisiting our magnet analogy, when we hold two ends with the same charge together, they repel; the same concept happens with fertilizers. This is why we frequently hear environmental challenges with phosphate or nitrate runoff from agricultural operations. These anions are quickly repelled out of the substrate because of the electromagnetic forces.
Revisiting our magnet analogy, when we hold two ends with the same charge together, they repel; the same concept happens with fertilizers. This is why we frequently hear environmental challenges with phosphate or nitrate runoff from agricultural operations. These anions are quickly repelled out of the substrate because of the electromagnetic forces.
Exchange between elements constantly occurs alongside particles. Some particles easily release nutrients, while others strongly bind them.
Drs. Shreckhise and Altland of USDA-ARS have demonstrated the ability to increase the phosphorus retention capacity of a substrate by amending with cheap and commercially available iron sulfate. As the iron sulfate dissolves, the positively charged iron acts as a bridge between the negatively charged phosphate and substrate particles. The iron-charged substrate will continue to capture and decrease phosphate leaching until these sites eventually become full.
When thinking of exchange capacity, we must be aware of the controlling factors: 1) pH; and 2) substrate surface area. Substrate pH plays a key role in nutrient availability; therefore, we spend a lot of effort adjusting substrate pH. But what are we actually changing? Basically, the pH of the substrate is an assessment of these exchange sites. Water (H2O) in the pores splits into H+ (a positively charged cation) and OH- (a negatively charged anion), which behave somewhat like fertilizer nutrients. These ions stick to substrate surfaces (on the exchange sites). Because these surface sites tend to always have an attached ion, when there are no nutrients in place, H+ and OH- fill these exchange sites.
As positively charged nutrients (cations) replace H+ ions on exchange sites, the H+ concentration of the pore water increases—this lowers pH. As negatively charged nutrients (anions) replace OH- on substrate exchange sites, the OH- concentration of the pore water increases and raises the pH. In essence, pH is a measure of the proportion of H+ and OH- ions that remain in the pore water. This is how the pH dictates nutrient availability—it’s the act of making the nutrients available by knocking them off the substrate surface to be accessible to the roots. Thus, the pH of the particle surface and the water inside the pore will dictate if the particle surface charge (positive or negative) and the specific ion (e.g., K+ or PO4-) are in the correct state or have the correct charge.
Substrates have little surface area when compared to mineral soils. This is due to the relatively large particles that dictate the amount of surface area and charges available for cations or anions to latch onto. Some substrate surfaces have a greater affinity for different ions—more dominant ions exchange for ions already on a given exchange site.
For example, a classic occurrence in pine bark substrates is for K+ ions to displace adsorbed Ca2+. However, most of the interactions that take place between the roots and fertilizer occur in the pore water solution. Thus, we don’t solely rely on cation or anion exchange capacity when growing in soilless substrates. Soilless substrates don’t contain previously stored nutrients, which often exist in mineral soils as a result of previous application and weathering. Therefore, we cannot count on any nutrients to be in adequate abundance and must supply all plant-required resources in soilless production.
Fertility is a dynamic process. It’s in part controlled by the substrate and their pores, regardless of using a water soluble or controlled-release fertilizer, to supply a continuous source of mineral nutrients in the right concentration. Irrigation and fertility are intrinsically linked and cannot be uncoupled. As such, water availability and mineral nutrient availability are also linked. Pore-water bathing the roots facilitates mineral nutrient uptake, transport of mineral nutrients across the concentration gradient and facilitates some exchange of ions with the substrate surface. The amount and location of mineral nutrient-laden water directly corresponds to nutrient availability.
By now, we should have a strong understanding of how water and nutrients move through the pores. It seems as if something important is missing. How many operations do you know just have containers filled with substrates without a plant growing in it? Probably not many. So why is this important? You’re correct—it’s all for the roots. Everything we do, whether it be fertilization or irrigation to substrate selection, is tailored for healthy root development. Make sure to check in next month for our last installation of the series that focuses on pores and roots. GT
Jeb Fields (jsfields@ufl.edu) is a Nursery Specialist with the University of Florida and the Editor-at-Large for the Nursery & Landscape Insider enewsletter. Kristopher Criscione (kscriscione@vt.edu) is a Nursery Specialist with Virginia Tech. Together, their work revolves around understanding and improving root zone management in plant production.