1/1/2025
Know Your Nitrogen
Josh Henry
Generally, nitrogen (N) can be categorized as being a synthetic form like nitrate (NO3), ammonium (NH4) or urea, or an organic form like amino acids. Another important way we categorize N is by its effect on substrate pH. Understanding each N form is an essential component to selecting the right fertilizer for your operation.
Synthetic vs. organic nitrogen
Inorganic and synthetic N sources are the primary forms used in professional greenhouse production. Most water-soluble fertilizers consist of some combination of NO3, NH4 and urea. Understanding which N sources are used in a commercial fertilizer and the ratio of those N sources can tell you a lot about how that fertilizer will affect factors including plant growth and substrate pH. For example, NH4-N promotes lush, “soft” growth with large leaves. Ammoniacal N also causes stem stretch and internode elongation, especially when applied in combination with phosphorus (P). It is important to note that P alone or in conjunction with NO3-N does not cause stretch. On the opposite end of the spectrum, NO3-N fertilization causes more compact growth, which is why NO3-N is typically considered the preferred form of N for floriculture production.
While urea itself is an organic molecule, the urea used in commercial fertilizers is produced synthetically, which is why it is not considered an organic N source. True organic N sources are often derived from plant, seed or kelp extracts, bone, blood or feather meal, or other composted materials. These sources cannot be used directly by plants and must first be converted or mineralized into the forms that plants can use, like NO3 and NH4. These organic N sources are typically converted to NH4 first, causing them to act more like NH4-N in terms of their effect on growth and substrate pH.
Nitrogen form and substrate pH
While substrate pH is largely controlled by irrigation water alkalinity and the initial quantity and quality of lime added to the substrate, N fertilization also plays an important role. The impact of N source is clear when reading any commercial water-soluble fertilizer label, which should have a statement regarding “Potential Acidity” or “Potential Basicity” given in pounds of calcium carbonate equivalent (CCE) per ton. The actual numbers associated with the potential acidity or basicity are not typically important to you, but simply show the degree to which a fertilizer will impact the substrate pH. These numbers are directly correlated to the percentage of NH4-N in the fertilizer with more NH4 providing a higher potential acidity and more NO3 providing a higher potential basicity.
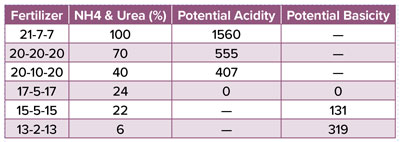 Table 1. Examples of common commercial water-soluble fertilizers and how the amount of ammonium- and urea-based nitrogen impacts potential acidity or basicity.
Table 1. Examples of common commercial water-soluble fertilizers and how the amount of ammonium- and urea-based nitrogen impacts potential acidity or basicity.
To demonstrate this concept, let’s consider a 21-7-7 fertilizer, which is often marketed as an “Acid Special” with a potential acidity over 1,500 lbs CCE per ton (Table 1). The N in this fertilizer is entirely from NH4-N or urea-N, which causes a drop in substrate pH. This occurs because NH4-N is positively charged, and the plant secretes a positively charged hydrogen ion (H+) to balance the equation, causing the pH to drop (Figure 1). Although the urea molecule is neutral and does not carry a charge, urea is typically converted to NH4 in the substrate, causing a subsequent drop in pH upon absorption. Conversely, NO3-N causes an increase in pH because it is negatively charged, and the plant releases a negatively charged hydroxide ion (OH-) to balance the absorption of NO3 (Figure 1).
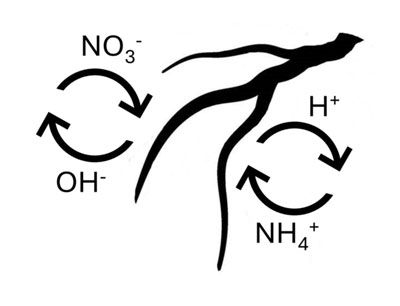
Figure 1. Graphical demonstration of the absorption of ammonium (NH4+) and nitrate (NO3-) ions, and the respective secretion of hydrogen (H+) and hydroxide (OH-) ions by the roots.
Other nitrogen concerns
While N deficiency is a more typical concern that comes with insufficient N fertility, it is important to also consider that NH4-N can cause toxicity. Symptoms of NH4 toxicity include lower leave marginal necrosis (browning) and leaf curl. Roots may also become necrotic starting at the tips. To avoid NH4 toxicity, use a high NO3 fertilizer with at least 60% NO3-N in warm conditions or 80% NO3-N when it is cool and cloudy.
Considering the N sources in a fertilizer is an often-neglected component of nutrient management and fertilizer selection, but understanding these basic principles is a powerful tool that you can use to manipulate growth and produce a healthy crop. GT
Josh Henry is a Technical Services Specialist for Ball Seed Company.