7/1/2023
Encarsia formosa: Biological Control Agent of the Greenhouse Whitefly
Dr. Raymond A. Cloyd

Encarsia formosa is a parasitoid of the greenhouse whitefly, Trialeurodes vaporariorum. E. formosa can be released into greenhouse production systems to manage greenhouse whitefly populations on ornamental and vegetable crops. This article discusses biology and behavior, commercial availability, use in greenhouses, and quality assessment associated with E. formosa.
Pictured: E. formosa adult female. All photos by Raymond Cloyd.
Biology and behavior
E. formosa adult females are 1/20 of an inch (1.0 millimeter) long with a dark brown to black head and thorax. Females have a yellow abdomen and translucent wings (Figures 1 and 2). Female E. formosa can reproduce through parthenogenesis, which is a form of reproduction where an egg can develop without being fertilized by a male.
A female uses her antennae to probe a whitefly nymph to assess suitability (based on size) for development of a larva. A single egg is laid into the whitefly nymph. Females prefer laying eggs into third and fourth instar whitefly nymphs because they’re large enough to accommodate development of the larvae. E. formosa females actively search for localized whitefly infestations and remain in areas where whiteflies are concentrated.
A single female can lay up to 15 eggs per day and up to 300 over her lifetime. A single egg is laid into each whitefly nymph. A female will not lay an egg into a whitefly nymph that’s been parasitized by another E. formosa female. A larva emerges (ecloses) from an egg and the life cycle continues.
There are three larval instars (stages between each molt). A whitefly pupa (fourth nymphal stage) parasitized by E. formosa eventually turns black (Figure 3), whereas a non-parasitized whitefly pupa is white or yellow with a T-shaped opening on the top. An adult E. formosa emerges by chewing a circular opening through the top portion of a dead whitefly pupa (Figure 4). E. formosa adults can emerge from parasitized whitefly pupae for up to two weeks.
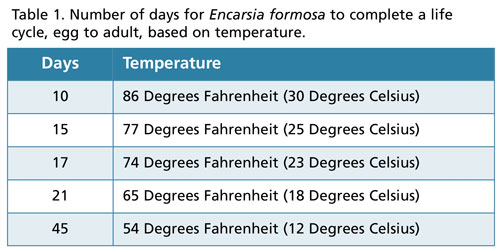
 The time required to complete the life cycle, from egg to adult, varies depending on temperature. The number of days to complete the life cycle based on temperature is shown in Table 1. E. formosa is most active at temperatures greater than 64F (17C). Optimal temperatures for managing whitefly populations are between 68 and 81F (20 and 25C) and relative humidity between 50% and 80%. E. formosa is more active during the day than at night. Short day lengths or photoperiods (less than 12 hours), temperatures below 64F (17C) and a relative humidity less than 50% can negatively affect the ability of E. formosa to manage whitefly populations.
The time required to complete the life cycle, from egg to adult, varies depending on temperature. The number of days to complete the life cycle based on temperature is shown in Table 1. E. formosa is most active at temperatures greater than 64F (17C). Optimal temperatures for managing whitefly populations are between 68 and 81F (20 and 25C) and relative humidity between 50% and 80%. E. formosa is more active during the day than at night. Short day lengths or photoperiods (less than 12 hours), temperatures below 64F (17C) and a relative humidity less than 50% can negatively affect the ability of E. formosa to manage whitefly populations.
Figure 3. Greenhouse whitefly pupa parasitized by E. formosa.
Figure 4. Greenhouse whitefly pupa with emergence hole associated with E. formosa adult.
Figure 5. Release card containing greenhouse whiteflies parasitized by E. formosa.
E. formosa females live from 10 to 30 days, however, adult longevity depends not only on temperature, but also on food availability. Females feed on honeydew, a clear sticky liquid produced by whiteflies, as a food source. In addition, females feed on the fluids exuded from wounds created by the ovipositor (egg-laying device). Furthermore, E. formosa females feed on second-instar nymphs and pupae to obtain nutrients for egg production.
Effects of plants on Encarsia formosa
Plant leaf hairs (trichomes) can inhibit the ability of E. formosa females to locate whitefly nymphs, thus reducing their ability to manage whitefly populations. E. formosa can manage whitefly populations on plants with few hairs, such as tomato (Solanum lycopersicum) and sweet pepper (Capsicum annuum), but not on cucumber (Cucumus sativus) plants where leaves have abundant hairs and thick leaf veins that inhibit movement. E. formosa females walk faster on leaves with few hairs, which increases their ability to locate whitefly nymphs.
Commercial availability and use in greenhouses
E. formosa can be purchased from suppliers of biological control agents as release cards (Figure 5) containing parasitized greenhouse whiteflies from which adults will emerge. There are approximately 50 parasitized greenhouse whiteflies per release card. Remove yellow sticky cards used to monitor whitefly adult populations before placing release cards within a crop to prevent capturing E. formosa adults. Two weeks after placing release cards within a crop, replace yellow sticky cards. Place release cards on the lower leaves of plants to reduce exposure to sunlight. Do not get the release cards wet when watering plants.
 Figure 6. Release card containing E. formosa inside Mason jar and yellow sticky square attached to underside of the lid.
Figure 6. Release card containing E. formosa inside Mason jar and yellow sticky square attached to underside of the lid.
Quality assessment
To determine the quality of product received from suppliers, follow the procedures below to verify that E. formosa adults are emerging from release cards. Sample five release cards from each shipment received from the supplier.
1. Place a single release card into a Mason jar. Attach a 1.0 x 1.0 in. (2.5 x 2.5 cm) yellow sticky square onto the underside of the lid using double-sided sticky foam (Figure 6).
2. Record the number of E. formosa adults captured on the yellow sticky square (Figures 7 and 8) after four, seven and 10 days.
3. Count and record the number of parasitized whitefly pupae per release card. Divide the number of E. formosa adults captured on the yellow sticky square by the total number of parasitized whitefly pupae per release card. Multiply by 100 to obtain percent emergence of E. formosa adults. Percent emergence of E. formosa adults from release cards should be greater than 80%.
E. formosa can effectively manage greenhouse whitefly populations in vegetable and ornamental cropping systems. For example, greenhouse producers can use E. formosa to manage greenhouse whitefly populations on tomato crops with minimal inputs from insecticide applications. GT
Dr. Raymond A. Cloyd is a Professor and Extension Specialist in Horticultural Entomology/Plant Protection at Kansas State University in Manhattan, Kansas. He can be reached at (785) 236-9150 or rcloyd@ksu.edu.