10/1/2019
Biocontrol Anecdotes
Albert Grimm
A whitefly control failure ranks among my worst job experiences. I’d just moved to Canada and landed my first “real job” in a tomato greenhouse. We’d taken a stab at whitefly control with biologicals long before bumblebees made it impossible to use pesticides in this crop. We had a young team, full of the kind of passion that wasn’t backed by experience.
Whitefly quickly won the contest. Plants were so infested that workers had to wear dust masks to avoid choking on insects. We had to invent a brand new method to wash honeydew from the fruit and the HID lamps caked up daily with corpses. When the lamps turned off, it began to “snow” whitefly flakes. We even rigged up a “whitefly vacuum” from several Shop-Vacs and a pipe-rail cart. The notion that things must get really bad before they can get better was of little comfort.
Today, biologicals have become our standard for whitefly control. They’re less expensive and more reliable than pesticides. It’s the most dependable of our control systems. For the last 15 years, we haven’t used insecticides on our poinsettias. Even in “bad whitefly years,” biocontrol produces clean crops. How did we get here?
Pesticide compatibility was the first learning curve. Our failure in tomatoes was most likely the result of incompatible pyrethroid sprays, which we’d used to clean up infestations at the end of crops. We thought that synthetic pyrethroids were rather benign and we didn’t realize that they were among the most persistent insecticides in a greenhouse environment.
Residues on the structure and in the soil can interfere with parasitoid wasps for many months. They’re particularly toxic to Encarsia formosa, our primary control agent for greenhouse whitefly. Once we stopped killing these beneficials, it became easy to control greenhouse whitefly on crops like fuchsia and lantana. Most of our growers no longer recognize them as whitefly hosts.
In poinsettias, however, whitefly control remained elusive. The trouble was threefold:
1. In poinsettia, the predominant whitefly pest isn’t the greenhouse whitefly, but Bemisia tabaci, the silverleaf whitefly.
2. In spring crops, the conditions for biologicals improve over time as days get longer and temperatures get warmer. Poinsettias, however, mature in cool weather and short days, which rapidly disfavor the biologicals, while whitefly continues to thrive.
3. Tolerable whitefly thresholds in poinsettia are extraordinarily low. Shipments are known to have been rejected for the presence of a few whitefly adults.
The low pest threshold combined with deteriorating conditions meant that whitefly had to be eliminated from the crop by late August. Residual populations couldn’t be allowed the time to reproduce into visible populations before the crop was ready to ship. Initial control attempts failed because our scouting methods were too crude to assess highly dispersed early populations of Bemisia. If we waited with control until whitefly was detectable by standard methods, we’d already run out of time.
We also lacked incentive because neonicotinoids had been working reliably well and it was difficult to make a case for the expense and risk of biological control. Not until Q-biotype Bemisia arrived did we learn that we can always attempt to rescue a crop with pesticides when biocontrol fails, but we can’t do the reverse.
An entire generation of growers learned instantly about the agony of pest infestations out of control. It was bad. Daily (!) applications of insecticides had no observable effect on whitefly populations. Tens of thousands of our poinsettias went to the compost. The experience was motivation enough to make whitefly biocontrol a priority in poinsettias. This is how we do it:
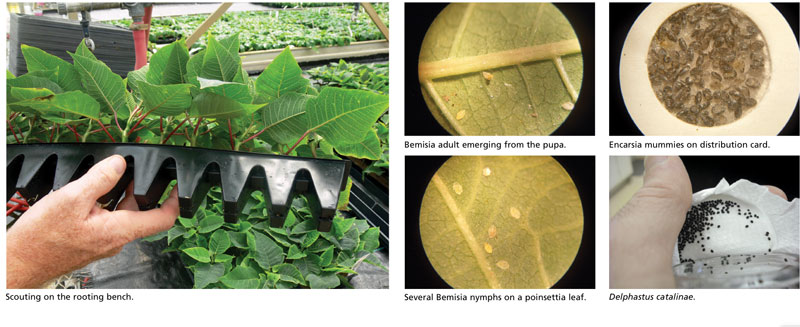
Scouting—Yellow sticky cards are much too crude for early whitefly scouting in poinsettia. We scout the plants themselves. We randomly pick plants, turn them upside down and examine the lower leaf surface for nymphs.
We start scouting very early. The first count is done on the rooting bench and weekly thereafter. We check each leaf on 100 cuttings per variety and we record the number of cuttings with ANY whitefly nymphs. If we find more than 10 leaves with nymphs, we count the number of individuals. This allows us to assess early whitefly pressure.
After transplant we continue with the same method. We randomly select 10 plants per bench and we scout the underside of every leaf. We still use yellow sticky cards, but we don’t use them for decision-making. We merely compare the results of our control efforts from year to year.
Overkill with parasitoid wasps—Don’t expect textbook black, parasitized mummies. There’s not enough time in a poinsettia crop to wait for parasitization. We inundate the crop with the expectation that Encarsia will kill the nymphs by host-feeding. In all those years, I only found five parasitized mummies in my poinsettias.
We begin to introduce Encarsia very early, in the first week after sticking while the cuttings are still under mist. We continue with weekly introductions until the start of short-day treatment. Pesticide residues are likely a problem for early introductions, but our rationale is that Encarsia will have to eat whitefly to absorb pesticides. If each wasp kills even just one nymph, Encarsia is still comparatively cheap.
With Eretmocerus mundus, such overkill strategies were even more effective. E. mundus was a game changer when it became available and it was our workhorse for 10 years of poinsettia biocontrol. E. mundus exclusively parasitizes Bemisia and it searches for highly dispersed nymphs. Encarsia prefers greenhouse whitefly, but will feed on Bemisia nymphs if they find them, albeit more sluggishly. Sadly, there are no longer any commercial insectaries that produce E. mundus.
 New control strategies—With E. mundus gone, Amblydromalus limonicus along with Encarsia has become the backbone of our poinsettia control. These predatory mites are comparatively expensive and we introduce them in three-week intervals at low rates from propagation through to start of short-day treatment. A. limonicus isn’t performing well unless it has a variety of food sources.
New control strategies—With E. mundus gone, Amblydromalus limonicus along with Encarsia has become the backbone of our poinsettia control. These predatory mites are comparatively expensive and we introduce them in three-week intervals at low rates from propagation through to start of short-day treatment. A. limonicus isn’t performing well unless it has a variety of food sources.
Consequently, we distribute brine shrimp cysts and frozen Ephestia eggs as supplementary food. A. limonicus also appears to be an effective predator of Lewis Mite and Echinothrips, two key poinsettia pests that can become problematic when we stop spraying broad-spectrum insecticides.
Delphastus catalinae is the third key biocontrol agent in our poinsettia program. These tiny ladybeetles feed exclusively on whitefly. They have excellent searching ability and they can clean up minor hotspots. They’re expensive, and in the absence of significant whitefly colonies, they won’t reproduce. They’re also exceedingly sensitive to pesticide residues. We introduce Delphastus in modest numbers in late September and early October for residual whitefly populations that have escaped the earlier barrage of beneficials. GT
Pictured: The author when he first started his growing career, trying to avoid choking on whiteflies.
Footnote: Several years ago, Agriculture Canada produced three short videos that describe our whitefly control programs: youtu.be/hdluQs6Jw-U; youtu.be/IlE1HVP1Abc; youtu.be/9nTqy-i9Ovw.
Albert Grimm is head grower for Jeffery’s Greenhouses in St. Catharines, Ontario, Canada.