8/1/2019
Improving Performance With Calcium
Katherine Bennett & James E. Faust
Over the past five years, we’ve been studying the use of calcium for building stronger, more resilient plants. This first article will discuss calcium uptake and why calcium concentrations in certain tissues may be different from what you expect. Next month’s article will cover the impact of calcium on leaf strength and how it plays a role in the plant’s susceptibility to Botrytis infection. Part three will dive into the different calcium sources and concentrations, and will describe the best application methods to improve the calcium concentration in leaves and flowers.
Calcium: The fundamentals
Calcium is a major plant nutrient that plays an important role in plant growth and development. Plants need calcium to increase strength of cell walls and to help decrease the plant’s susceptibility to pathogen invasion.
Calcium is considered to be an immobile nutrient, meaning calcium is fixed in older tissues and the older leaves don’t remobilize calcium to support the newly developing leaves. Because of this immobility within the plant, deficiency symptoms first appear in the new growth of the plant, e.g., the immature leaves.
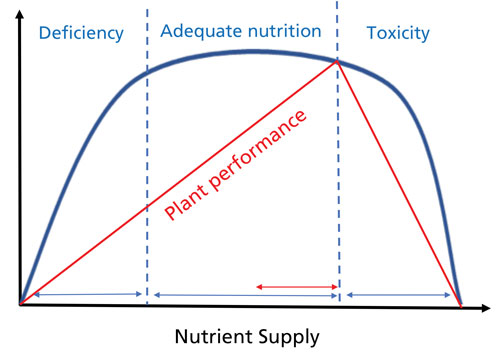 Figure 1. The BLUE line displays the conventional response to plant nutrients, such as calcium. This response is based on the visual appearance of the plant. Note the very broad range of nutrients supplied that will provide adequate nutrition for a healthy-looking plant. The RED line indicates that higher nutrient concentrations provide improved plant performance up to a point. In other words, benefits in plant performance may be observed at higher nutrient rates even though plant appearance doesn’t change. The performance benefits of higher calcium nutrition can be improved tissue strength and resistance to Botrytis infection.
Figure 1. The BLUE line displays the conventional response to plant nutrients, such as calcium. This response is based on the visual appearance of the plant. Note the very broad range of nutrients supplied that will provide adequate nutrition for a healthy-looking plant. The RED line indicates that higher nutrient concentrations provide improved plant performance up to a point. In other words, benefits in plant performance may be observed at higher nutrient rates even though plant appearance doesn’t change. The performance benefits of higher calcium nutrition can be improved tissue strength and resistance to Botrytis infection.
The classic symptoms of calcium deficiency are necrosis and/or distorted new growth, such as puckered or cupped leaves, and burnt leaf or flower margins. Calcium deficiencies are also common in large plant organs, such as the fruit of tomatoes and watermelons that display blossom-end rot.
Calcium toxicities aren’t typically a problem, however calcium is a positively charged ion, called a cation, that competes for cation exchange sites with other cations, such as potassium, ammonium and magnesium. Thus, calcium application rates must be properly balanced with the other cations, otherwise imbalances can occur.
A generalized response to nutrient supply is shown in Figure 1—Note the difference in the visual appearance (blue line) and plant performance (red line) responses. A wide range of nutrients supplied to the plant will result in normal-looking plants; however, higher concentrations of those nutrients in the tissues may produce beneficial responses. In the case of calcium, tissue strength and resistance to Botrytis infection occurs at higher tissue concentrations even though the visual appearance of the plant may not be different.
Calcium uptake
To understand calcium nutrition, we need to appreciate how calcium is unique amongst the essential plant nutrients. Calcium uptake and movement through the plant begins with the movement of water into the roots. Energy isn’t required for calcium movement into the root and movement into the plant occurs along with the uptake of water. This is termed “passive uptake.” All of the other macronutrients are actively taken up by the plant, i.e., the plant expends energy to transfer the nutrients from the surrounding soil into the roots.
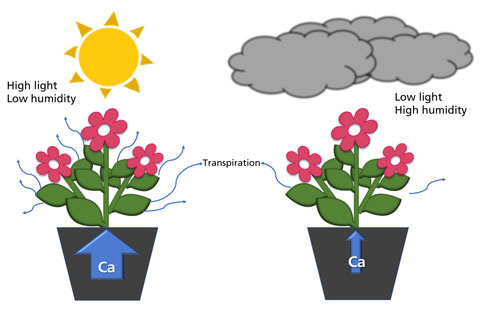 Figure 2. Examples demonstrating the effect of the environment on transpiration and calcium uptake. High light and low humidity (left) generate a high transpiration rate and low calcium uptake, while low light and high humidity (right) result in a low transpiration rate and low calcium uptake.
Figure 2. Examples demonstrating the effect of the environment on transpiration and calcium uptake. High light and low humidity (left) generate a high transpiration rate and low calcium uptake, while low light and high humidity (right) result in a low transpiration rate and low calcium uptake.
Once in the plant, calcium moves through the xylem with the flow of water from the roots to the leaves as transpiration pulls water throughout the plant. Although present in the phloem, calcium is largely immobile, resulting in low rates of calcium movement into fruits and flowers, which are primarily supplied with nutrients through the phloem. Since transpiration directly affects water uptake, calcium uptake is also affected by the environmental factors that impact transpiration. This contrasts with the other macronutrients in which uptake occurs due to the expenditure of energy by the plant and thus isn’t limited by transpiration rates.
Several environmental factors influence transpiration, which affects calcium uptake and distribution in plants. Cool temperatures, cloudy weather and rain create a high humidity situation that reduces transpiration and consequently reduces calcium uptake (Figure 2).
Increased humidity within the plant canopy can also influence the movement of calcium into new shoots. For example, in head lettuce, leaf-tip burn is caused by a localized calcium deficiency in the leaf margin. Increasing air circulation helps to decrease the humidity in the plant canopy and thus creates higher transpiration rates which, in turn, pulls more calcium into the immature leaves.
Calcium distribution in plant tissues
Flowers typically have much lower transpiration rates compared to leaves. For example, our measurements showed that petunia leaves transpire 7.5 times more water than petunia flowers. Since flowers don’t do photosynthesis, they don’t need to take in carbon dioxide, therefore flowers lack stomata. As a consequence, much less opportunity exists for importing calcium into flower petals than into leaves.
Many nutrients delivered to developing flowers occur through the phloem; however, calcium is largely immobile in the phloem, making calcium movement into flower tissue even more difficult. Tissue analysis of petunia flowers and leaves shows that the leaves typically contained 2% to 3% Ca (dry weight basis), while the flower petals contained just 0.2% to 0.3% Ca, a ten-fold difference.
Calcium fertilization practices
In general, calcium can be supplied to plants through a combination of four methods:
1. Calcium can supplied in a water-soluble fertilizer that may contain calcium nitrate or calcium chloride. The calcium rates typically supplied in the fertigation solution during container production range from 30 to 150 ppm Ca, while hydroponic growers typically apply 100 to 200 ppm Ca. Commercial fertilizers can range from providing 0% Ca, such as in 20-10-20, to 11% Ca in 15-0-15. Complete fertilizers, such as 13-2-13 and 15-5-15, typically contain 5% to 6% Ca. The calcium concentration is always reported on the fertilizer label.
2. Calcium can be incorporated into the growing media in the form of limestone (dolomitic, calcitic or hydrated), bonemeal, fishmeal or gypsum (calcium sulfate). These products will slowly release calcium into the growing media over time.
3. Calcium is naturally occurring in well water and the concentrations can range wildly from 0 to 180 ppm Ca. A water test is necessary to identify the calcium concentration in your water supply. It’s important to have this measurement when designing your calcium fertilization program.
4. Calcium can be sprayed onto plant tissues with one of several sources, including calcium chloride, calcium nitrate and others. Sprays are most beneficial for boosting the calcium concentration in low-transpiring tissues, such as flowers and fruits. Spray applications have historically been made weekly on poinsettias in the 400 to 500 ppm Ca range to prevent bract-edge burn. In the coming articles in this series, we’ll demonstrate the benefit of using much higher concentrations of calcium during spray applications.
Calcium fertilization has great potential for enhancing plant performance at a very low cost. Next month’s article will elaborate on the effect that calcium can have towards improving the leaf strength of cuttings and improving the resistance of flowers to Botrytis infections. GT
Acknowledgements: The American Floral Endowment and the Floriculture Research Alliance support our research efforts exploring practical techniques to improve Botrytis management.
Katherine Bennett recently completed her M.S. degree at Clemson University and is currently a Research Scientist at Metrolina Green-houses. James Faust is an Associate Professor at Clemson University.