3/1/2018
Minding Your pHs and Qs
Silvia Valles Ramirez & James Altland
Greenhouse and nursery growers are keenly aware of the importance of substrate pH. It affects nutrient availability in container crops and some crops are more sensitive to pH than others. For example, petunias, geraniums, hydrangea, boxwood and red maples are just some crops that have very specific pH requirements due to their sensitivity to certain nutrient availabilities.
Substrate pH is affected by many factors, including substrate components, lime rate, fertilizer type and even the crop itself. One of the most influential factors affecting substrate pH is the irrigation water. Irrigation water pH and alkalinity are two factors often monitored by nursery growers so they can better manage substrate pH. It’s critical to differentiate between irrigation water pH and substrate pH. Irrigation water pH is a measure of the acidity in the irrigation water supply, while substrate pH is the measure of the acidity in the substrate solution.
Substrate pH is ultimately what we’re concerned about. Substrate pH directly affects nutrient availability. So how does irrigation water pH affect substrate pH? You might be surprised to learn it has very little impact—irrigation alkalinity is far more important.
What is pH?
Technically speaking, water pH is the negative logarithm of the hydrogen (H+) ion concentration in water. (A chemist might argue that H+ ions do not exist and we should really discuss hydronium (H3O+) ions, but for the sake of clarity, we’ll continue with the traditional model of using H+.) Don’t worry too much about the logarithm part—just remember that pH is really measuring the H+ ion concentration in water. The logarithm is only used to express the concentration (which is a really small number) in a form that’s easier to write and understand.
The chemical notation for water is H2O, however, water doesn’t exist solely as H2O. A very small portion of the H2O molecules will split into hydrogen (H+) and hydroxyl (OH-) ions. Imagine a glass of pure water (Figure 1, left glass). Water is never pure; there are always dissolved minerals (especially in ground water) or dissolved gasses that affect water pH and its chemistry. But for the sake of explanation, imagine this glass of water is absolutely pure.
In this glass, 1 in 10 million H2O molecules will split into H+ and OH- ions. In this pure water, the number of H+ ions are equal to the number of OH- ions and pH is neutral with a value of 7. Any time something is added to water and reacts within the water, it can cause a shift in the concentration or balance between H+ and OH- ions.
If a reaction in water causes an increase in the H+ ion concentration (Figure 1, middle glass), the solution becomes more acidic and the pH drops (to less than 7). Likewise, if something is added to the water that decreases the H+ ion concentration (Figure 1, right glass), the solution becomes more basic (pH is greater than 7).
What is alkalinity?
Alkalinity is the concentration of bicarbonates in a solution. Be careful not to confuse the terms alkaline and alkalinity; alkaline is an adjective describing a solution with high pH (above 7), while alkalinity is a noun that refers to the bicarbonate concentration of a solution.
Alkalinity is determined by measuring the concentration of carbonates (CO32-), bicarbonates (HCO3-) and hydroxyls (OH-) in solution. Other chemicals can contribute to alkalinity, however, carbonates and bicarbonates are the primary components.
Irrigation water with high alkalinity will tend to raise the pH of a soil or container solution over time. When irrigation water with a high concentration of HCO3- is applied to a container substrate, the HCO3- reacts with H+ to form H2O and CO2 (HCO3- + H+gH2O + CO2). The CO2 from this reaction is a gas and will dissipate into the atmosphere. The net effect from this reaction is that H+ ions are removed from the solution. Recall our example with the three glasses of water—any reaction that reduces the concentration of H+ will cause an increase in pH. This is why alkalinity in water raises substrate pH.
Case study
Consider the following experiment where we irrigated plants with one of three different irrigation treatments: We used 6-in. diameter pots filled with a substrate composed of 80% pine bark and 20% sphagnum peat moss. The substrate was amended with a controlled release fertilizer (Osmocote 15-9-12) incorporated at 13 lb./cubic yard and potted with a single rose liner (Rose Radrazz). Containers were irrigated with either reverse osmosis (RO) water, a 0.0001 mM potassium hydroxide (KOH) solution or a 0.005 M potassium bicarbonate (KHCO3) solution. There were six containers per irrigation type. Substrate pH was recorded monthly over three months using the pour-through procedure.
 The RO water had a pH of 6.26 and very low alkalinity (Table 1). The RO water from our lab is very pure, although not as pure as the hypothetical example we gave above in reference to the three glasses of water. Nonetheless, it’s pure enough to have a pH close to 7 and very low alkalinity. The water with KOH had a high pH (due to the addition of OH- ions from the KOH), but still very low alkalinity.
The RO water had a pH of 6.26 and very low alkalinity (Table 1). The RO water from our lab is very pure, although not as pure as the hypothetical example we gave above in reference to the three glasses of water. Nonetheless, it’s pure enough to have a pH close to 7 and very low alkalinity. The water with KOH had a high pH (due to the addition of OH- ions from the KOH), but still very low alkalinity.
Finally, the water with KHCO3 had a similar pH to the KOH water, but note that its alkalinity was high. The value of 275 mg/L CaCO3 is a little higher than well water in many parts of the U.S., which typically ranges from 100 to 200 mg/L CaCO3.
Substrates irrigated with RO or KOH had similar pH values throughout the study (Figure 2), despite the fact that KOH water was almost 2 pH units higher than RO water. Even though the KOH water had a much higher pH, its very low alkalinity, similar to RO water, meant that it had no effect on substrate pH.
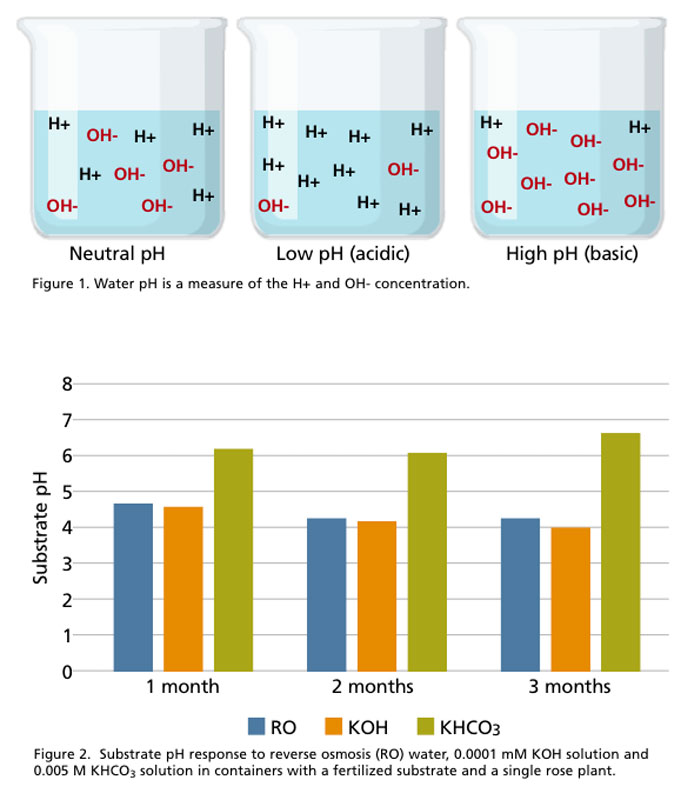 Furthermore, substrate pH in these two treatments declined (albeit slightly) from one month to three months. On the other hand, substrates irrigated with KHCO3 had higher substrate pH throughout the three months. While the KHCO3 and KOH water had nearly identical pH, the final substrate pH in the KHCO3 treatment was 2.5 units higher than the KOH due to the influence of alkalinity. These data demonstrate that irrigation alkalinity, and not irrigation pH, affect substrate pH in containers over time.
Furthermore, substrate pH in these two treatments declined (albeit slightly) from one month to three months. On the other hand, substrates irrigated with KHCO3 had higher substrate pH throughout the three months. While the KHCO3 and KOH water had nearly identical pH, the final substrate pH in the KHCO3 treatment was 2.5 units higher than the KOH due to the influence of alkalinity. These data demonstrate that irrigation alkalinity, and not irrigation pH, affect substrate pH in containers over time.
Even though the substrate pH varied across the three irrigation treatments, the roses in all these containers were healthy and vigorous with no differences in growth or flowering. While not discussed in this case study, we also measured the impact of the rose plants themselves on substrate pH, in which case roses had little or no impact. The dominant factor in changing substrate pH was irrigation water alkalinity.
Why is alkalinity more important than pH?
The reason irrigation alkalinity is more important than irrigation pH is due to relative concentration. Recall that pH is a measure of the H+ ion concentration in water. Because of this, we can calculate the actual H+ and OH- concentrations at any given pH. We typically don’t discuss H+ or OH- concentrations because the numbers are very small and require a lot of decimal points. But for the sake of explanation, let’s go through an example of just how insignificantly small these concentrations are.
Recall that OH- ions, like those added to the KOH water, react with H+ ions and essentially remove them from the solution. The concentration of OH- ions in a pH 6 solution (close to our initial RO water pH) is 0.00000001 mg/L, while the concentration of OH- in a pH 8 solution (like our KOH water) is higher, but still only 0.000001 mg/L. The bicarbonates associated with irrigation water alkalinity raise pH by also causing a decrease in the H+ ion concentration. The alkalinity in most irrigation water throughout the U.S. is 100 to 200 mg/L, and in our case study, was 275 mg/L. That’s quite a larger number!
So whether we’re talking about a lower pH solution (pH 6) or a relatively higher pH solution (pH 8), the concentration of OH- ions that might neutralize H+ in container substrate solutions is insignificantly small compared to the H+ neutralizing ability of bicarbonates. This is why irrigation alkalinity affects substrate pH, but irrigation pH has virtually no impact.
So what’s the takeaway message? Understanding and maintaining substrate pH over time is necessary to produce high-quality crops, but be sure to measure irrigation water alkalinity to better predict how substrate pH will change over time. GT
Silvia Valles Ramirez is a visiting scientist at the Ohio State University. Dr. James Altland is a Research Horticulturist with the USDA-ARS in Wooster, Ohio. He can be reached by email at james.altland@ars.usda.gov. Mention of any commercial product in this article is for educational purposes only and should not be considered an endorsement by the Ohio State University or USDA-ARS.