11/26/2013
Keeping it Green
Chris Currey, Roberto Lopez, VIijay Rapaka, Jim Faust & Erik Runkle
Unrooted geranium cuttings have a short post-harvest life and low tolerance to high temperatures during shipping. Undesirable shipping conditions can increase respiration (reducing carbohydrates) and increase ethylene generation in geranium cuttings, which can cause lower-leaf yellowing and senescence during propagation. Additionally, abscised leaves can host botrytis and cause losses during propagation. Therefore, fungicides are often applied during propagation and infected leaves are removed during production to reduce pathogen problems.
Applications of plant growth regulators (PGRs) such as benzyladenine (BA; a cytokinin) and/or gibberellic acid (GA) may suppress lower-leaf yellowing and senescence. Growers producing Easter lilies are already familiar with applying a BA and GA, known commercially as Fascination or Fresco, to keep the older, lower leaves green. In the past few years, some propagators of zonal geraniums have also been utilizing BA and GA during propagation to reduce lower-leaf yellowing of geranium cuttings. Our objectives were to:
1) determine if BA and/or GA should be applied either before or after shipping;
2) evaluate whether rooting hormones could overcome reduced rooting caused by the PGR applications; and
3) quantify the effects of BA + GA applications on several geranium cultivars.
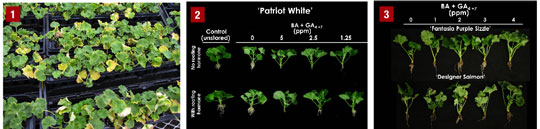 Figure 1. Geranium cuttings with lower-leaf yellowing are a common sight during propagation.
Figure 1. Geranium cuttings with lower-leaf yellowing are a common sight during propagation.
Figure 2. Patriot White geranium cuttings 20 days after being treated with 0 to 5 ppm BA + GA4+7 and rooting hormone (with or without) after a simulated shipping.
Figure 3. Fantasia Purple Sizzle and Designer Salmon geranium cuttings 28 days after being treated with 0 to 4 ppm BA + GA4+7.
What we did
Experiment 1—Application timing. Cuttings of Patriot White geraniums were harvested from vegetative stock plants. Cuttings were placed in a plastic tray and PGR applications were made before or after simulated shipping (pre-shipment or post-shipment, respectively). The PGR applications consisted of sprays containing a surfactant and 2.5 or 5 ppm BA (MaxCel; Valent BioSciences Corporation), 0.5 or 2.0 ppm GA3 (ProGibb; Valent), 2.5 or 5.0 ppm each of BA + GA4+7 (Fascination; Valent), or water and surfactant only, all at a volume of 2 qt. per 100 sq. ft. The simulated shipping treatment consisted of placing cuttings in zip seal polyethylene bags that were packed in a shipping box, which was then placed in a dark chamber to simulate shipping. Following simulated shipping, cuttings were stuck in 10-cell propagation strips containing a peat and perlite mix and rooted in a greenhouse under mist and air and substrate temperatures of 73F (22C).
Seven days after cuttings were stuck, each cutting was subjectively evaluated using a scale from 1 to 5 (1 = poor, 5 = excellent) based on overall leaf color and senescence. Twenty days after cuttings were stuck, the total number of senesced leaves and whether the cutting was “pullable” when removed from the propagation tray were recorded. Roots and shoots were separated and dried to measure dry weights.
Experiment 2—Rooting hormone application. Cuttings of Patriot White geranium were harvested and placed into simulated shipping as described previously except for a non-stored control. Following simulated shipping, the end of each cutting was either briefly dipped in a solution containing 1,000 ppm IBA + 500 ppm NAA (Dip ‘N Grow Liquid Rooting Concentrate) or received no rooting hormone. Sprays containing a surfactant and 1.25, 2.5 or 5.0 ppm BA (MaxCel), 1.25, 2.5 or 5.0 ppm each of BA + GA4+7 (Fascination), or 0.25, 0.5 or 2.0 mg·L‒1 GA3 (ProGibb) were applied to cuttings. The propagation environment, culture and data collection were as previously described.
Experiment 3—Cultivar screen. Cuttings of Designer Salmon, Fantasia Purple Sizzle, Fantasia Pink Shell and Presto Dark Red zonal geraniums were received from a commercial propagator and dipped in a rooting hormone solution as previously described. Cuttings were then placed in 72-cell propagation trays in the greenhouse. Sprays containing a surfactant and 0, 1, 2, 3 or 4 ppm each of BA + GA4+7 (Fresco; Fine Americas) were applied immediately before placement under mist. The “greenness” of lower leaves was measured seven days after the beginning of propagation with a SPAD meter, and the number of senesced leaves was recorded 28 days after cuttings were treated and placed in the greenhouse. Shoot and root dry weights were measured following harvest.
What we observed
Experiment 1—Application timing. In general, applying BA, GA3 or BA+GA4+7 after simulated shipping increased the visual quality rating of Patriot White geraniums. However, the effectiveness depended on the PGR. Cuttings that weren’t treated with PGRs were visually rated 2.2 or 3.1 for cuttings with or without a simulated shipping, respectively. The visual ratings were 1.9 to 3.0 for cuttings treated before the simulated shipping, while the visual ratings of cuttings treated after the simulated shipping were 4.9 or 5.0. This indicates that post-shipment PGR applications inhibited leaf senescence more than pre-shipment applications. Additionally, cuttings treated post-shipment were more pullable than cuttings treated before shipment. The highest concentrations of BA, BA + GA4+7 and GA3 substantially reduced rooting (by at least 84%) compared to stored, untreated cuttings. The inhibition of rooting was greater with pre-shipment treatments than post-shipment applications.
Experiment 2—Rooting hormone application. Seven days after the PGRs were applied and cuttings were placed in the greenhouse under mist, the visual rating of Patriot White geranium cuttings was 2.4 for untreated, stored cuttings. The visual rating for cuttings treated with BA + GA4+7 (1.25 or 2.5 ppm) or GA3 (0.25 or 0.5 ppm) was 5.0. Cuttings treated with 5.0 ppm BA + GA4+7 or 0.5 and 2.0 ppm GA3 were less pullable than untreated, stored cuttings, although the percentage of pullable cuttings increased 16% to 52% when they were dipped in the rooting hormone.
Experiment 3—Cultivar screen. Cuttings of Fantasia Pink Shell and Presto Dark Red geranium did not exhibit any leaf yellowing, indicating that not all cultivars are susceptible to lower leaf yellowing. However, both Designer Salmon and Fantasia Purple Sizzle displayed leaf yellowing once propagation began.
Seven days after PGR applications, the “greenness” of Fantasia Purple Sizzle and Designer Salmon increased as the concentration of BA + GA4+7 increased from 0 to 4 ppm. After 28 days in propagation, the total number of senesced leaves decreased with increasing PGR concentration for Fantasia Purple Sizzle. Designer Salmon cuttings treated with at least 1 ppm BA + GA4+7 had fewer senesced leaves than untreated cuttings. Shoot and root dry weights were not affected by PGR applications.
The take-home message
Products containing BA + GA4+7 are most likely the best for commercial use in geranium propagation. Our results indicate that applying PGR solutions after shipping were more effective than the same PGR applications made before shipping. BA and GA inhibited rooting, as observed in Experiment 1, however dipping cuttings in a rooting hormone partially overcame that suppression, as demonstrated in Experiment 2.
Finally, geranium cultivars vary in their susceptibility to lower-leaf yellowing and senescence, and there was no negative effect of BA + GA4+7 applications on the cultivars that didn’t exhibit leaf yellowing. This could allow growers to apply BA + GA4+7 to cuttings of all geranium cultivars in propagation, thus simplifying management decisions.
Using PGRCALC, we estimated the PGR spray cost for a foliar application of solution containing 2.5 to 5 ppm BA + GA4+7 at a rate of 2 qt. per 100 sq. ft. to be $0.44 to $0.88 per 1,000 sq. ft. of bench space. Based on this calculation, we believe that the costs for PGR and application labor to prevent lower-leaf senescence are minimal when compared to the potential labor costs to manually remove leaves, plus any losses from botrytis-infected cuttings.
GT
The authors would like to thank Fine Americas, Valent BioSciences, Ball Horticultural Company, The Paul Ecke Ranch, and J.R. Peters for supporting this research.
Chris Currey (ccurrey@iastate.edu) is an assistant professor at Iowa State University, Roberto Lopez (rglopez@purdue.edu) is an associate professor and Extension specialist at Purdue University, Vijay Rapaka (vrapaka@smithersoasis.com) is manager of grower research at Smithers-Oasis Company, Jim Faust (jfaust@clemson.edu) is an associate professor at Clemson University, and Erik Runkle (runkleer@msu.edu) is an associate professor and Extension specialist at Michigan State University.