6/15/2009
Water pH and Pesticide Activity
Raymond Cloyd
When a pesticide fails to control or regulate an insect or mite population in your greenhouse, there’s a common tendency to blame it on resistance. However, there are a number of other factors that may actually be responsible, including poor coverage, waiting too long between applications, using the wrong pesticide, using the wrong label rate, improper timing (applying pesticides when susceptible life stages such as larvae, nymphs, and adults aren’t present), using an old (more than 10 years) pesticide and lastly, the pH of the water or spray solution.
Nearly all growers are aware of and understand the importance of pH in regard to water quality and growing medium. The pH (potential hydrogen) refers to a logarithmic scale from 1 to 14. A pH of 7 is neutral, whereas a pH below 7 is acidic and above 7 is basic (alkaline). It’s important to understand that pH is a logarithmic scale (e.g. a pH of 6 is 10 times more acidic than a pH of 7) and the sensitivity of a pesticide to water pH will increase by a factor of 10 for every pH unit.
Although you will (or should) assess the pH of your irrigation water and growing medium, it’s just as important to test the pH of your water or spray solution, as it can influence the management of insect and mite pests with pesticides (both conventional and alternative).
A reduction in pesticide effectiveness, in general, may be due to hydrolysis, which is a chemical process whereby molecules are cleaved into several smaller components by the addition of water molecules. The rate of hydrolysis is dependent on 1) pH, 2) pesticide chemistry, 3) length of time in the spray tank and 4) water temperature in the spray tank.
Pesticides may undergo alkaline hydrolysis in which a pH greater than 7 causes some pesticides to endure a chemical degradation process. The alkaline water breaks apart or fragments pesticide molecules, resulting in the release of individual ions (electrically charged atoms), which may then reassemble with other ions. These new combinations may not have any insecticidal or miticidal activity, thus reducing or compromising the effectiveness of a pesticide spray. However, some pesticides may actually undergo acid hydrolysis (pH below 7.0). In addition, the length of exposure in an alkaline spray solution is critical. As such, what comes out of the spray nozzle during the first hour of a spray application could be more effective than what comes out in the last hour of a spray application.
Furthermore, an 18 degree (10C) increase in the temperature of the spray solution will double the rate of decomposition. At a pH of 9 and water temperature of 77F (25C), acephate (Orthene) loses 50% of its activity in approximately one to two days. In fact, doubling the temperature will double the speed of the degradation process. The exposure of the spray tank to sunlight will also impact the rate of hydrolysis. It should be noted that even pesticide mixtures (tank mixes) or combining two or more pesticides together may lead to an increase in a pesticides’ susceptibility to decomposition.
Pesticide manufacturers usually have data associated with the effect of water pH on the half-life of certain pesticides. Half-life is the time required for 50% of the active ingredient to hydrolyze or break down, or it’s the length of time in which the pesticides’ original strength is reduced by 50%. In general, insecticides are more susceptible to alkaline hydrolysis than either fungicides or plant growth regulators. Insecticides in these chemical classes are most sensitive to alkaline hydrolysis or “high” pH water solutions: organophosphate (acephate and chlorpyrifos), carbamate (methiocarb) and pyrethroid (bifenthrin, cyfluthrin, fenpropathrin, fluvalinate and lambda-cyhalothrin). However, some pesticides aren’t affected by water pH, such as fenbutatin-oxide (ProMite).
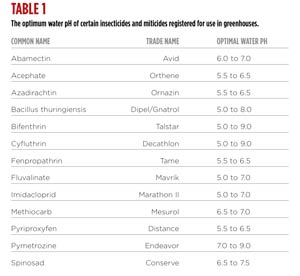
It’s essential to monitor the spray solution pH and adjust accordingly in order to maximize pesticide effectiveness. The “ideal” pH range of most insecticides and miticides is between 5.0 and 6.5; however, several insecticides perform better at or above a pH of 7. Table 1 is a listing of some insecticides and miticides and the optimum water pH.
It’s possible to correct the pH of the water, although the process should be performed carefully. The use of pH paper isn’t the most accurate means of monitoring the pH within 0.5; however, since a pH between 6.0 and 7.0 is generally acceptable, the use of pH paper is valid.
So what should you use to adjust the pH of the water? Well, acetic acid (vinegar) is readily available and can be added to water in the spray tank in small increments; check periodically with pH paper. It’s important to avoid adding too much vinegar since the water pH should be around 6.5. To increase the pH, add household ammonia. Finally, be sure to adjust the pH of the water prior to adding any pesticides to the spray tank.
You can also adjust pH with buffers or water-conditioning agents, which are compounds that reduce the potential for alkaline hydrolysis and modify the pH of the spray solution so that it’s easier to maintain within a range of 4 to 6. These compounds are safer to use than materials such as sulfuric acid when lowering the spray solution pH. However, it’s important to add any buffers to the spray tank before adding pesticides, because certain pesticides may begin to breakdown as soon as they come into contact with an alkaline solution.
You can avoid water pH reducing the effectiveness of a pesticide spray by following these principles:
1) Follow manufacturer label directions associated with the appropriate water pH. Below are examples from labels indicating the importance of water pH.
• Ornazin (azadirachtin): Product will break down in spray tank mixtures that have pH values exceeding 7.0. Recommended pH range: 5.5 to 6.5.
• Floramite (bifenazate): Product has been shown to degrade rapidly when mixed and/or stored with alkaline water or high temperatures (122F or 50C). To prevent degradation under alkaline conditions, solutions must be used promptly. Alternatively, a commercially available buffering adjuvant can be added to the solution to reduce the pH to a neutral/acidic range.
• Mavrik (fluvalinate): Buffer spray water to pH of 5 to 7, if necessary.
• Mesurol (methiocarb): Performance may be reduced when the spray solution has a pH greater than 7. The pH of the spray solution must be corrected by the addition of a suitable buffering or acidifying agent for optimum activity.
2) Regularly test water pH because the pH of water can change during the growing season. Water samples should be collected in a clean, non-reactive container such as a glass jar. Be sure to collect a water sample that’s representative of the spray solution. Allow the water to run long enough so that water standing in the spray hose is flushed-out. Determine the pH of the water solution with pH paper immediately after collection.
3) Apply spray solution as soon as possible after mixing. It’s recommended to use a pesticide spray solution (or mixture) within six hours or less to avoid potential problems associated with pH.
4) Adjust water pH with buffers or water-conditioning agents. These are compounds that can inhibit the process of alkaline hydrolysis and modify the pH of the spray solution in order to maintain the pH within the desired range. These compounds are much easier and safer to use than attempting to lower the water and spray solution pH with materials such as sulfuric acid. In addition, acetic acid (vinegar) may be used to acidify water.
Before deciding that resistance or other factors are responsible for inadequate control of insect and mite pests, always check water pH, since this may be the actual culprit. Monitoring water pH will help you maintain the effectiveness of pesticides in your greenhouses. 
Raymond A. Cloyd is associate professor and extension specialist in ornamental entomology/IPM, Department of Entomology, Kansas State University, Manhattan, Kansas. E-mail: rcloyd@ksu.edu.