What the ... ?
I was in Homestead, Florida, two weeks ago, for an introductory trip in my new job with SePRO. I visited several growers and researchers in that area and enjoyed the pore-opening and sweat-inducing power of heat and humidity (reminded me of Malaysia).
I was awe-struck by the size of the nursery industry in Homestead. I was a post-doctoral researcher working on biological control of invasive pests in South Florida 17 years ago. The nursery industry around Homestead was growing at the time, but today I couldn’t recognize the place. The industry seems to have tripled or quadrupled in size and output in the past 20 years!
It’s no joke to say that Florida is the capital of invasive species. I worked on pink hibiscus mealybug, papaya mealybug and Asian citrus psyllid during my tenure in South Florida. There are many more invasive insects that came to settle in Florida after I left.
I see these invasive species whenever I turn my head. I found one of them on a black olive tree (no, not the true olive, but Terminalia buceras, AKA oxhorn bucida) planted in front of the hotel I stayed at. What is this?


Additional Solutions for Thrips parvispinus
One of the main purposes of my visit to Homestead is to see for myself the impact of infestation by Thrips parvispinus in the area. I’ve seen the serious damage these thrips can cause. Reading and writing about a problem is nowhere near enough to express the visual and emotional impact of seeing and facing the problem in person.
What’s heartbreaking is the sense of helplessness and anxiety many growers have, whether they expressed that outwardly or not. Many simply call this species the “super thrips.” We know so little about the biology, ecology and management of this thrips. Where (around the nursery) do they come from? Why were they damaging this block or this cultivar and not that block or that cultivar? What can be used to reduce the population and prevent them from infesting my plants? Is there any biological control solution? How best to develop a rotation program when I have to spray a limited number of effective products once or twice a week? How to I keep the plants clean and pass inspection? How do I prevent my nursery from being quarantined?

Alexandra Revynthi and Lance Osborne from the University of Florida and Zee Ahmed and Cindy McKenzie from USDA-ARS are leading the charge in developing solutions for Thrips parvispinus. They've developed a website to serve as an information sharing hub on this pest. You’ll find host plant, biology, management information, an awesome scouting sheet and lots of pictures on this website.
Research updates and webinars given by the researchers also appear on this website. For example, a tHRIve webinar (organized by the Horticultural Research Institute) presented on May 10 is linked on this website.
A recent update on the control of this thrips species, presented on May 4 and posted on Alexandra’s website, is also linked. Alexandra’s group has recently identified additional insecticides that show good efficacy against Thrips parvispinus (data are presented in the update).
When sprayed directly, XXpire (spinetoram + sulfoxaflor) and Conserve (spinosad) were effective in killing adults AND nymphs, whereas Timectin (abamectin; many generics) and Hachi-Hachi (tolfenpyrad) were effective against nymphs. The fresh residue of XXpire, Conserve, Timectin, Hachi-Hachi, Piston (chlorfenapyr; likely also Pylon; greenhouse use only), Kontos (spirotetramat) and Pedestal (novaluron) were effective against nymphs. Fresh residue of XXpire, Conserve, Piston and Overture (pyridalyl; greenhouse use only) were effective in killing adults. To prevent feeding by nymphs, XXpire, Conserve, Timectin, Piston, Kontos, Sarisa (cyclaniliprole), Acephate (acephate), Hachi-Hachi and Mainspring (cyantraniliprole) should be used. XXpire, Conserve, Timectin, Sarisa, Acephate and Overture can reduce adult feeding.
I look forward to additional research and identification of effective tools from colleagues in Florida. Only with more information can we develop a more integrated approach to the management of Thrips parvispinus.

Watch for Spotted Lanternfly Egg Hatch
Folks, it’s time to watch for the hatching of spotted lanternfly (SLF) eggs and develop a plan for management, if it's needed. According to the prediction map by Penn State University (PSU) Extension, egg hatch is upon us in Virginia and getting closer in the other infested states. In the PSU prediction map, the percent of probability of egg hatching is indicated by color—areas of darker color have a greater chance of hatching.
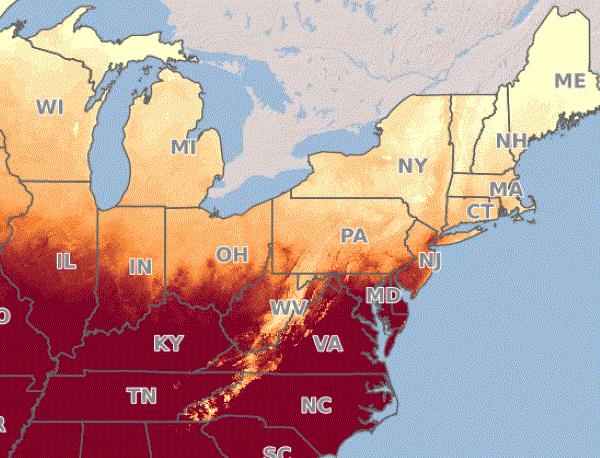
Source: PSU
If, for some reason, you don’t want to use the PSU map, you can also look at another prediction tool offered by the Network for Environment & Weather Application (NEWA), with collaboration from the New York State IPM Program (NYSIPM) at Cornell University and several land-grant universities. You can select the closest weather station and insert the first date you’d found an egg mass in 2022. The program will then provide you with a prediction of the development status of the SLF population near you. Just for fun, I looked at the status at Bridgeport, Connecticut, using the default egg observation date of September 15. There are about 87 degree-days remaining to go before egg hatch occurs in this area.
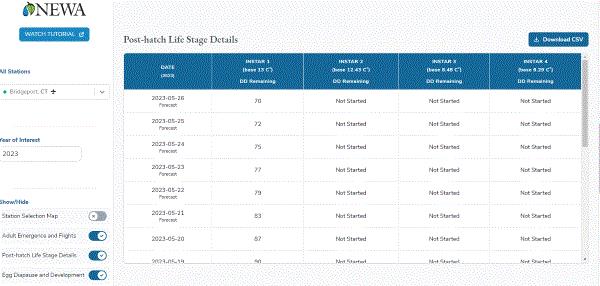
Source: NEWA
Remember that, although the PSU map shows you the entire country and the NEWA tool allows you to choose from a lot of weather stations, that doesn’t mean SLF has established in all these places. The map and prediction are generated without considering where the pest occurs, so don’t freak out!
According to the distribution map generated by NYSIPM, as of May 15, infestation is present in parts of Connecticut, Delaware, Indiana, Maryland, Massachusetts, Michigan, New Hampshire, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Rhodes Island, Virginia and West Virginia. (This list doesn’t include states where sightings have been reported, but establishment hasn’t been confirmed.)

The Inaugural Biosolutions Guide
The 2023 GrowerTalks Biosolutions Guide has been published, adding to GrowerTalks’ offering of Grower Guides. Edited by our own Paul Pilon, this resource provides information from industry experts on the uses and efficacy of various biological pest management products. The Biosolutions Guide is divided into three sections: Bioinsecticides, Biofungicides and Biocontrols.
Carlos Bográn of OHP discussed the types and uses of bioinsecticides. A great part of Carlos’ article is a table comparing the advantages and disadvantages of bioinsecticides and conventional insecticides, which will give you food for thought. An efficacy table is included in this section, giving you a quick view of the use rate, use sites, efficacy against target pests, etc.

Ann Chase of Chase Agricultural Consulting contributed to an article on the myths, considerations and challenges of using biofungicides. As in the bioinsecticide section, a biofungicide efficacy table anchors in the biofungicide section.
Suzanne Wainwright-Evans of Buglady Consulting headlined the biocontrol section and shared her experience of working in the biocontrol and horticultural industry. You’ll find hard-learned lessons that may be useful in your own biocontrol program. You’ll also find Suzanne’s “cheat sheet” of the characteristics and uses of biological control agents useful. Carlos and Ann close the section by sharing their recommendations on how to integrate bioinsecticides and biofungicides with biocontrol.
I’m sure you’ll find this new BioSolutions Guide useful. You can have a look at it by clicking HERE. And a printed copy will be mailed with the June issue of GrowerTalks.

Answer to “What the … ?”
The circular deposition of wax (which gives this species its common name) and the location (southern Florida) are pretty good clues for identifying today’s mysterious creature. The black olive tree is infested with the rugose spiraling whitefly, along with other pests I didn’t ask about. Here's a picture of nymphs (along with a couple of mealybugs) from the same tree:

A native of Central America, the rugose spiraling whitefly was first found in Miami-Dade County in 2009, a couple of years after I left Florida to join Clemson University. In some ways, I’m kind of glad that I didn’t have to mess with this pest. The infestation became more noticeable a few years after the first detection and began to attract media attention. Folks were freaking out and thought the end of some favorite tree species (such as gumbo limbo, black olive, avocado, palms, banana, etc.) was at hand. The actual host list of the rugose spiraling whitefly isn’t as long as it seems. The adults can settle and feed on many plant species, but these are not true hosts (i.e., plant species that can support complete development).
Nonetheless, the whiteflies seemed to be everywhere and feed on everything. The white wax covers the underside of leaves of some plants, not to mention the copious amount of honeydew and sooty mold associated with the infestation. Immeasurable efforts were spent on spraying and drenching trees and shrubs throughout central and southern Florida.
Obviously, the whitefly is still around these days, but the infestation doesn’t seem as serious as it was. Perhaps folks are used to the idea of having a few white rings on the leaves and don’t think much about treating them. Perhaps some native (or introduced) parasitic wasps and predators finally caught up to it and keep the whitefly population under control. The rugose spiraling whitefly joins the rank of invasive pest species that have faded slowly and became part of the local fauna over time.
Click HERE for a factsheet about the rugose spiraling whitefly.




See y’all later!
JC Chong
Technical Development Manager at SePRO
Adjunct Professor at Clemson University
This e-mail received by 27,847 subscribers like you!
If you're interested in advertising on PestTalks contact Kim Brown ASAP!