3/1/2018
If Superman Were a Substrate Scientist
Paul C. Bartley III, Dr. Brian E. Jackson & Dr. Bill Fonteno
In our previous article in the February issue of GrowerTalks, “A New Look at Substrates,” we discussed a need researchers have to nondestructively collect spatial and temporal data and how imaging equipment, such as Computed Tomography (CT), have demonstrated considerable promise for the observation and characterization of horticultural substrates within a container.
Indeed, adopting medical imaging equipment, such as CT or Magnetic Resonance Imaging (MRI), for horticultural research may prove to be immensely insightful, as it removes the barriers for visual observation and enables us, like Superman, to see through walls. However, instead of using this equipment as physicians to evaluate the ailments and pains that plague us, we in the Horticultural Substrates Lab at North Carolina State University are seeking to evaluate how plants develop, grow and thrive.
X-ray vision, for plants
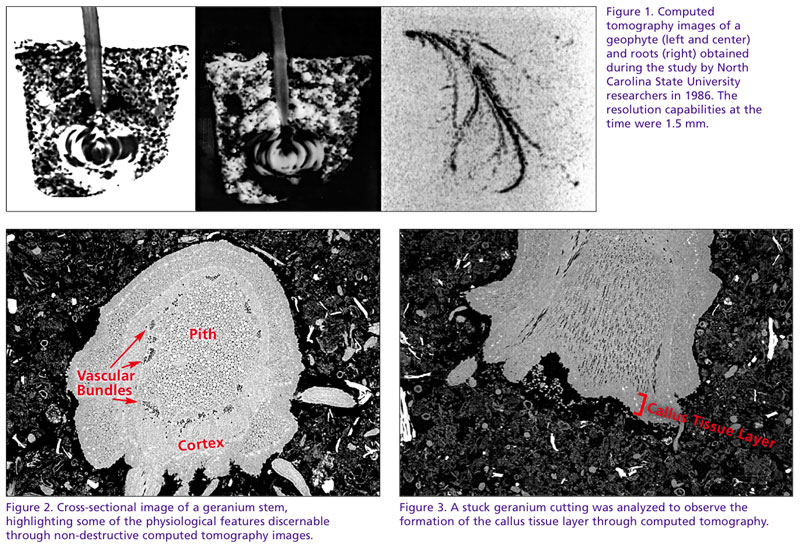
The first to use X-ray tomography for horticultural interest was a group of NC State University researchers in 1986. Published a year later, the objective of the work was to observe and assess the distribution of water held within horticultural foam substrates. During the study, attempts were made to visualize geophytes growing in containers (Figure 1).
Since the first foam block or geophyte was scanned, tomography has progressed considerably, allowing researchers from the University of California-Davis, Cornell University and others to visualize and quantify the effect of soil compaction and plant-to-plant interactions within a container. As imaging technology continues to show promise in sensitivity, cost effectiveness and availability, it’s now, more than ever, well placed to contribute significantly to studying plant growth and development effects from seed to flower.
Our industry’s drive to increase production efficiency and sustainability has forced us, as researchers, to expand on our understanding of plant growth dynamics. From the work of dedicated researchers in our industry, we understand that there are numerous stimuli that can alter the development and growth of plants.
These effects can be exogenous, pertaining to external factors, such as the growth effects of temperature differentials or the effect of spectral quality (light) on stem elongation. They can also be endogenous, pertaining to internal factors, such as carbohydrate content or plant hormones and their effect on vegetative propagation.
Plant CT scans
Without a doubt, the systems we manage are vastly complex with numerous influential factors, which the grower must avoid or utilize to effectively control the development and growth of plants. It’s within these dynamic systems that the ability to non-invasively evaluate plant performance has tremendous upside.
This “non-invasive” attribute of tomographic research is particularly important when studying sensitive stages of plant growth and development, such as the healing of graft unions or root development of vegetative cuttings. During these wounding and healing stages of plant development, it can be helpful to identify physiological responses to wounding and to monitor the plant’s subsequent
regeneration.
Just as a physician can evaluate internal organs, CT imaging gives us the ability to observe the internal organs of plants—such as the cortex, vascular bundles and pith—through cross-sectional images (Figure 2). Masses of dedifferentiated cells or callus tissues in plants are indicators of regeneration, an essential process for root development of vegetative cuttings (Figure 3). The ability to observe the formation of callus tissue and subsequent root growth without sample disturbance could help us better understand the influence external and internal factors impose on the processes of root initiation.
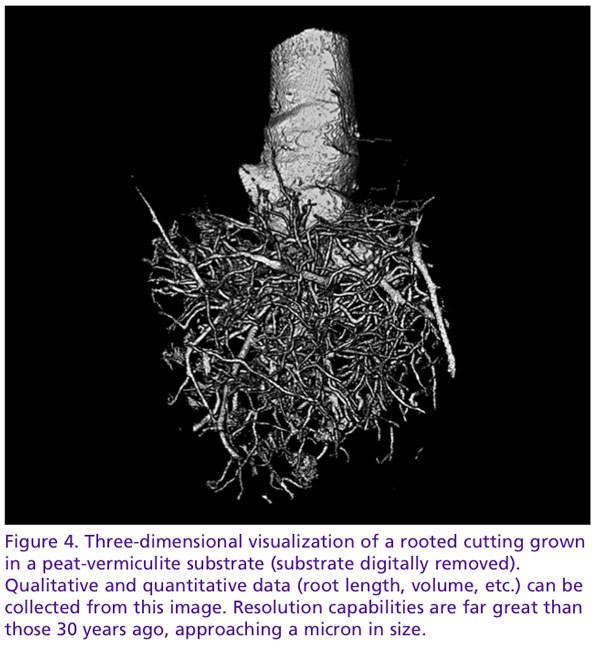 Equally as important as the study of wound regeneration is the study of root growth and architecture, the primary organ for water and nutrient uptake. We understand root growth to be dynamic, dependent on many chemical and biological factors. Though our understanding of these interactions is well developed, how they interact with their immediate physical environment, the substrate, remains as much a mystery. As horticulture offers the unique possibility to design substrates in relation to the crop’s requirements, continuous monitoring of root growth in this dynamic relationship is desirable.
Equally as important as the study of wound regeneration is the study of root growth and architecture, the primary organ for water and nutrient uptake. We understand root growth to be dynamic, dependent on many chemical and biological factors. Though our understanding of these interactions is well developed, how they interact with their immediate physical environment, the substrate, remains as much a mystery. As horticulture offers the unique possibility to design substrates in relation to the crop’s requirements, continuous monitoring of root growth in this dynamic relationship is desirable.
How do substrates affect root development? It’s a question researchers have asked for decades. To answer this, we’ve created containers with clear walls (NCSU’s Horizotrons) and painstakingly hand-washed root balls. While these methods have been effective, they lack the ability to observe three-dimensional interactions between the root and substrate. How do roots interact to pores of varying dimensions? Do they bulldoze their way with brute force through small pores or follow a path of least resistance, gently weaving their way between particles? Tomography may give us the unique perspective to observe pore-root interactions in a way never before possible.
Just as substrates may affect root development and architecture, the physical and hydraulic properties of substrates may be affected by the exploration and expansion of plant roots. If we design a substrate for the ideal characteristics for the moment a seed, cutting or plug is sown, are those characteristics the same a week later? A month later? Does your management of that system need to change in order to compensate for the substrate’s change in properties?
These are important questions to ask in the quest to design and engineer substrates for more intensive, productive crop systems. To aid in this design, we need the ability to see through objects, to analyze our substrates, the spaces they create, the water they capture and their dynamic interaction with plant roots.
Thanks to the partnership with the Shared Materials and Instruments Facility at Duke University, we’re a step closer to understanding what it would be like if Superman were a substrate scientist. GT
Paul Bartley is a doctoral student in the Horticultural Substrates Laboratory at North Carolina State University and can be reached at pcbartl2@ncsu.edu. Dr. Brian E. Jackson is an Associate Professor in the Department of Horticultural Science at NC State University (Brian_Jackson@ncsu.edu). Bill Fonteno is a professor in the Department of Horticultural Science at NC State University (bill_fonteno@ncsu.edu).